Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6475
Peer-review started: June 26, 2020
First decision: September 12, 2020
Revised: September 22, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: November 7, 2020
Postoperative delayed bleeding (PDB) after gastric endoscopic submucosal dissection (ESD) is the most common adverse event in patients receiving antithrombotics even with second-look endoscopy. Moreover, with the increasing prevalence of cardiovascular and cerebrovascular diseases in an aging population with associated lifestyle-related diseases, an increasing number of patients receive antithrombotics. Several attempts have been made to prevent PDB in aging population; however, a consensus has yet to be reached.
To examine the efficacy of third-look endoscopy (TLE) for PDB prevention.
One hundred patients with early gastric neoplasms receiving antithrombotics were prospectively enrolled and subjected to ESD with TLE between February 2017 and July 2019. The primary endpoint was PDB rate, which was compared with our preset threshold. Furthermore, we divided the bleeding period into early-and late-onset PDB (E-PDB and L-PDB, respectively) and analyzed its rate. As a secondary analysis, we compared PDB rates with those of a historical control group, using propensity score matching, and calculated the PDB rates per antithrombotic agent use in each group.
In total, 96 patients and 114 specimens were finally evaluated. The overall PDB rate was 7.9% (9/114) [90%CI: 4.7-13.1, P = 0.005], while the late-and early-onset PDB rates (L-PDB and E-PDB) were 5.3% [90%CI: 2.7-9.9, P < 0.0001] and 2.6% [90%CI: 1.1-6.4, P = 0.51], respectively. Propensity score matching generated 58 matched pairs for TLE and control groups. No differences were found in overall PDB incidence (10.3% vs 20.7%, P = 0.12), whereas L-PDB occurrence significantly differed (5.2% vs 17.2%, P = 0.04) between groups. Considering antithrombotics’ use, the overall PDB rate was higher for direct oral anticoagulants and multiple antithrombotics in the control group, while L-PDB incidence was lower in the TLE group for these agents (8.7% vs 23.1% and 5.0% vs 29.4%, respectively).
TLE for gastric ESD reduces overall PDB, and especially L-PDB incidence, among patients receiving antithrombotics.
Core Tip: The major adverse event after endoscopic submucosal dissection for early gastric cancer under antithrombotic therapy is post-operative delayed bleeding (PDB). We verified the effectiveness of third-look endoscopy (TLE) before discharge in a phase II trial. Our results suggest that TLE significantly reduced PDB incidence among patients who continued to receive antithrombotic drugs. We concluded that TLE which is manageable for every endoscopist is a simple and effective method for preventing PDB under antithrombotic therapy.
- Citation: Ikeda R, Hirasawa K, Sato C, Ozeki Y, Sawada A, Nishio M, Fukuchi T, Kobayashi R, Makazu M, Taguri M, Maeda S. Third-look endoscopy prevents delayed bleeding after endoscopic submucosal dissection under antithrombotic therapy. World J Gastroenterol 2020; 26(41): 6475-6487
- URL: https://www.wjgnet.com/1007-9327/full/v26/i41/6475.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i41.6475
Despite the widespread eradication of Helicobacter pylori and improvements in treatment, both the morbidity and mortality of gastric cancer remains high, especially in Asia[1,2]. As a minimally invasive approach, endoscopic treatment for early gastric cancer (EGC) has dramatically advanced with the development of endoscopic submucosal dissection (ESD). Additionally, the advent of ESD has fulfilled the promise of en bloc and complete EGC resection[3]. The removal of a complete specimen by ESD is directly associated with the final pathological evaluation and greatly contributes to curability[4]; therefore, ESD is now the gold standard for treatment for EGC; however, postoperative delayed bleeding (PDB) is a major adverse event of gastric ESD, which cannot be eliminated. The overall frequency of PDB is reported at 5.1%-5.7%[5-8].
We previously reported that antithrombotic therapy (ATT) is an independent risk factor for PDB; in particular, we found that anticoagulation and combination ATT are associated with late-onset PDB[5,6]. Recently, with the increasing prevalence of cardiovascular and cerebrovascular diseases in an aging population with associated lifestyle-related diseases, an increasing number of patients receive ATT[9]. Although several attempts to prevent PDB in these patients have been reported, a consensus has yet to be reached[10-12].
In our previous reports, second-look endoscopy (SLE) on postoperative day (POD) 1 was performed in all cases of gastric ESD[5,6]. Therefore, we anticipated that the addition of third-look endoscopy (TLE) could prevent PDB in patients under ATT; as such, we attempted to verify its efficacy in this prospective study.
This study was designed as a phase II trial in a single center and was approved by the Clinical Ethics Committee of Yokohama City University Graduate School of Medicine, conforming to the Helsinki Declaration. The study is registered in the UMIN Clinical Trials Registry System (000025607). All patients provided written informed consent before undergoing ESD. We enrolled patients with EGC receiving antithrombotic agents, and TLE was added to conventional ESD, including SLE, in all participants.
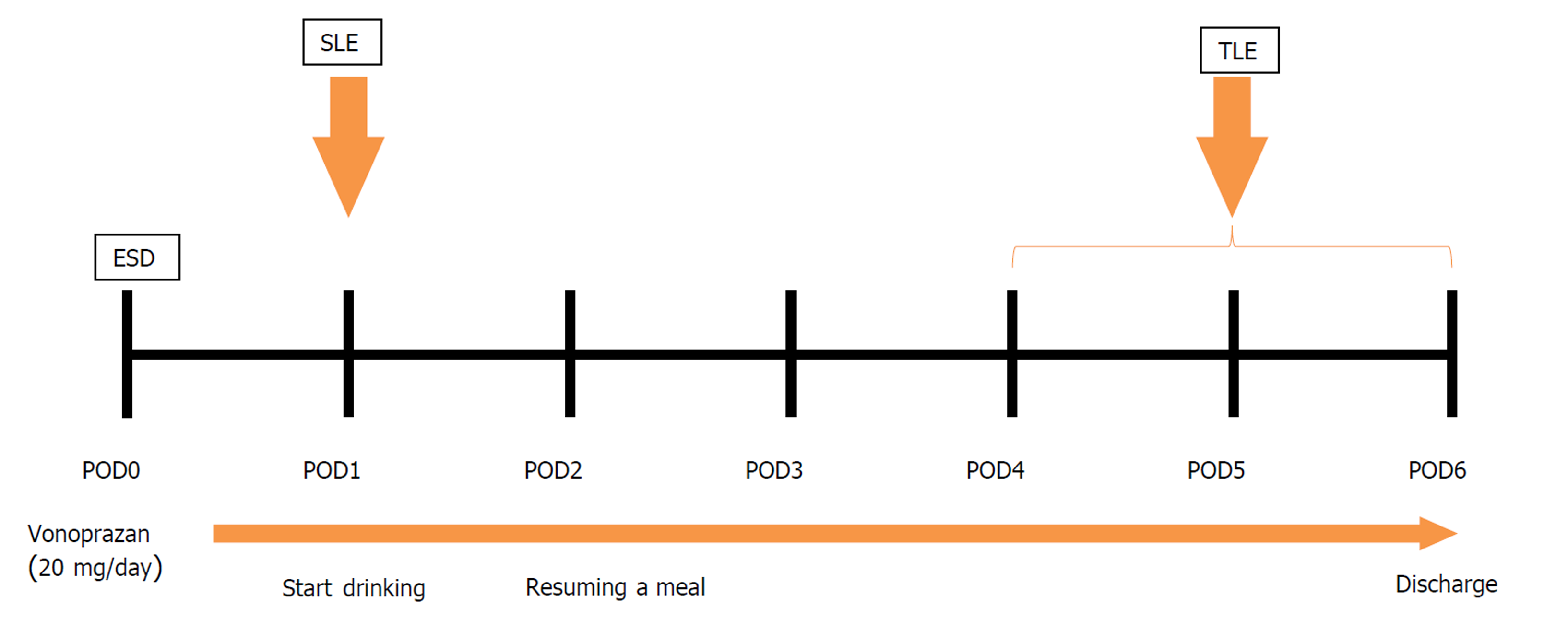
The flow chart of the study protocol is shown in Figure 1. Patients received an oral potassium-competitive acid blocker (vonoprazan, 20 mg/d) on the day before the ESD. After ESD treatment (POD 0), SLE was performed on POD 1 to check the condition of the artificial ulcers; if blood oozing or exposed blood vessels (Forrest classification 1b, 2a) were confirmed, prophylactic hemostasis for electrocoagulation was provided. Fluid intakes were resumed 2 h after SLE, and liquid meals were reinstated on POD 2. TLE was performed on POD 5, and endoscopic hemostasis was augmented the same as SLE. If POD 5 was a hospital holiday, we performed TLE either on the previous or the next day (POD 4 or POD 6). Patients without any adverse events were discharged on POD 6. The potassium-competitive acid blocker was continued for at least eight weeks after ESD.
The primary endpoint was the overall PDB rate in patients with TLE. Additionally, we divided the bleeding period into early and late-onset PDB (E-PDB: bleeding before POD 5; L-PDB: bleeding after POD 6, respectively) as a sub-analysis of the primary endpoint. As a secondary analysis, we compared the PDB rate with a historical control group of 109 patients (132 specimens) subjected to conventional ESD under ATT in our institution from January 2014 to January 2017, using propensity score matching to adjust the baseline characteristics of the two groups. Finally, we calculated the PDB rates per antithrombotic agent used in each group.
We enrolled 100 consecutive patients > 20 years old with early gastric neoplasms receiving antithrombotic agents between February 2017 and July 2019. The indication for gastric ESD included: (1) Adenoma and intestinal-type mucosal cancer lesions of any size without ulcers; (2) Differentiated mucosal cancer lesions ≤ 3 cm with ulcers; (3) Undifferentiated mucosal cancer ≤ 2 cm in size without ulcers[13,14]; and (4) no evidence of either lymph node or distant metastasis confirmed by preoperative computed tomography. Patients were excluded if they: (1) Had lesions in the remnant stomach and underwent type B-1 or 2 reconstruction distal gastrectomy and gastric tube reconstruction after lower esophagectomy; (2) Had infectious diseases requiring systemic treatment; (3) Were either pregnant or possibly pregnant; (4) Had comorbidities such as psychiatric disorders and dementia that made it difficult to confirm their intention to participate; (5) Had unstable angina developed within the last 3 wk or myocardial infarction within 6 mo; (6) had respiratory disease requiring continuous oxygen administration; (7) Had uncontrolled hypertension; (8) Had uncontrolled diabetes; (9) Had anemia requiring blood transfusion (hemoglobin < 7.0 g/dL), a bleeding tendency (platelets < 50000); and (10) Were judged to be inappropriate for this study by the attending physician.
The antithrombotic agents included in this study were the following: Antiplatelet agents (aspirin, thienopyridine, and cilostazol), anticoagulants [warfarin and direct oral anticoagulants (DOAC)], and multiple antithrombotic agents [e.g., dual antiplatelet therapy (DAPT), antiplatelet and anticoagulant agents], and were categorized accordingly. To determine drug continuation or withdrawal and resumption, we consulted the attending physician of the specialty department before planning the procedure and determined the feasibility of drug withdrawal and its duration according to the Japan Gastroenterological Endoscopy Society (JGES) guidelines[15,16]; however, our drug-specific retrospective large dataset indicated that antiplatelet agents do not significantly increase PDB rates after gastric ESD[6], and that patients taking anticoagulants or DAPT have a high thrombotic risk. Additionally, the evidence level in JGES guidelines regarding the possibility of continuation and withdrawal of ATT is not so high. Therefore, we continued ATT as long as possible. If a patient discontinued an antithrombotic agent, we reinstated oral treatment from POD 2 onwards following SLE. Patients receiving warfarin were hospitalized the day before the ESD, and we confirmed the prothrombin time-international normalized ratio, thus adjusting warfarin dose to increase the activated partial thromboplastin time to 1.5-2-times that of the pretreatment value. Heparin bridge therapy (HBT) was never used to substitute anticoagulant therapy or DAPT because of the poor evidence regarding thrombosis prevention and a higher PDB rate than that in our previous data[6,16,17].
All patients underwent pretreatment endoscopic examinations using endoscopes that provide narrow-band imaging with magnifying endoscopy (GIF-H260Z or GIF-H290Z; Olympus Medical Systems Corporation, Tokyo, Japan) to accurately confirm tumor margins. All procedures were performed under conscious sedation using midazolam or propofol and pentazocine. A single-channel endoscope with water jet (GIF-Q260J; Olympus, Tokyo, Japan) or a 2-channel multi-bending endoscope (GIF-2TQ260 M; Olympus) was used with a high-frequency power supply unit (VIO300D, ICC200; ERBE, Tübingen, Germany or PSD60; Olympus, Tokyo, Japan) for electrocoagulation. Gastric ESDs were performed using a conventional procedure. After marking around the tumor and injecting saline or 10% glycerin solution mixed with sodium hyaluronate (MucoUp; Johnson & Johnson Medical Company, Tokyo, Japan), we started mucosal incision using a Dual knife (Olympus Medical Systems, Co. Tokyo. Japan) and performed submucosal dissection using insulated-tip knife-2 (Olympus Medical Systems, Co. Tokyo. Japan) and Dual knife. The remaining visible blood vessels in the ESD artificial ulcer were cauterized using hemostatic forceps (Coagrasper; Olympus Medical Systems Co. Tokyo, Japan). After resection, we dispersed a mixture containing aluminum hydroxide gel, liquid magnesium hydroxide, and 10000 U thrombin (approximately 100 mL).
PDB was defined as an episode of hematemesis and/or melena, or a decline in hemoglobin levels of ≥ 2 g/dL, and requirement of emergency endoscopic hemostasis. PDB occurring before POD 5 was defined as E-PDB, whereas that occurring after POD6 was defined as L-PDB.
The sample size was calculated based on our historical dataset. The PDB rate of consecutive patients who were subjected to gastric ESD under ATT from January 2014 to December 2015 was 15.3% (16/104). Therefore, we assumed a threshold of 15% for the overall PDB rate and hypothesized that this rate would be reduced to 7% by the TLE intervention. To achieve an 80% power with a one-sided significance level of 0.05, we calculated that 92 cases were required. Considering the feasibility of the study, the final sample size was calculated at 100 patients.
We analyzed the baseline characteristics and outcomes using the χ2 or Fisher exact tests for categorical data. For the primary analysis, we calculated the PDB rate and its 90% confidence interval. If the upper limit was less than 15%, then our primary hypothesis was met. For the secondary analysis, we used propensity score matching to compare the TLE group with the historical control group. To balance bias, we used logistic regression including the following factors for calculating the propensity score: age, sex, location, morphology, specimen size, ulcer, tumor depth, pathology, procedure time, tumor size, and antithrombotic agents. P < 0.05 was considered to indicate statistical significance. JMP (version 12) software package (SAS Institute, Cary, NC, United States) was used for statistical analyses.
One hundred patients with 126 early gastric neoplasms under ATT were subjected to ESD. Several synchronous multiple lesions were removed in the same specimen, and since the study analysis was conducted based on specimens obtained by ESD, 100 patients, and 118 specimens were investigated; of which four were excluded because of intraoperative perforation. Therefore, 96 patients and 114 specimens were finally evaluated. We used a specimen-based instead of patient-based analysis because, on a patient’s basis, it is difficult to determine which specimen should be prioritized based on clinicopathological characteristics. Moreover, if a patient with multiple lesions is excluded, it is not possible to perform analysis following the actual clinical practice.
Baseline clinicopathological characteristics are shown in Table 1. The participants included 98 males and 16 females aged between 59 and 88 years (mean 75.5 ± 0.6 years). Regarding the tumors’ location, 18.4% (21/114), 12.3% (14/114), and 69.3% (79/114) of the tumors were in the upper, middle, and lower stomach, respectively, while 8.8% (10/114), 31.6% (36/114), 40.3% (46/114), and 19.3% (22/114) were in the anterior wall, posterior wall, lesser curvature, and greater curvature, respectively. Regarding tumor morphology, 42.9% (49/114) of the tumors were of the protruded type and 57.0% (65/114) of the flat/depressed type. The median tumor and specimen sizes were 11.0 mm (range 2-70) and 35.0 mm (range 18-95), respectively. Histologically, 95.6% and 4.4% of the tumors were differentiated and undifferentiated adenocarcinomas, respectively. No adenomas were included during the study period. Deep submucosal invasive carcinomas were recognized in 1.8% (2/114) and ulceration was confirmed in 5.3% (6/114) of the tumors. Synchronous multiple occurrence of lesions was found in 22.9% (22/96) of all patients.
| TLE group (n = 114) | TLE group (n = 114) | ||
| Age, mean ± SD (yr) | 75.5 ± 0.6 | En-bloc resection, % (n) | 100 (114) |
| Sex | R0 resection, % (n) | 100 (114) | |
| Male, % (n) | 86.0 (98) | Curative resection, % (n) | 90.4 (103) |
| Female, % (n) | 14.0 (16) | Median procedure time (range) | 41.5 (10-180) |
| Location-1 | SLE Hemostasis, % (n) | 68.8 (77) | |
| Upper, % (n) | 18.4 (21) | Forrest classification 1b, % (n) | 10.7 (12) |
| Middle, % (n) | 12.3 (14) | Forrest classification 2a, % (n) | 58.0 (65) |
| Lower, % (n) | 69.3 (79) | TLE Hemostasis, % (n) | 35.7 (40) |
| Location-2 | Forrest classification 1b, % (n) | 10.7 (12) | |
| Anterior wall, % (n) | 8.8 (10) | Forrest classification 2a, % (n) | 25.0 (28) |
| Posterior wall, % (n) | 31.6 (36) | The day of TLE, % (n) | |
| Lesser curvature, % (n) | 40.3 (46) | POD 4 | 29.8 (34) |
| Greater curvature, % (n) | 19.3 (22) | POD 5 | 54.4 (62) |
| Morphology | POD 6 | 14.0 (16) | |
| Protruded, % (n) | 42.9 (49) | Comorbidities | |
| Flat/depressed, % (n) | 57.0 (65) | Hypertension, % (n) | 85.1 (97) |
| Median tumor size (range) | 11.0 (2-70) | Diabetes mellitus, % (n) | 21.9 (25) |
| Median specimen size (range) | 35.0 (18-95) | Hyperlipidemia, % (n) | 54.4 (62) |
| Synchronous occurrence, % (n) | 22.9 (22/96 patients) | Cardiac disease, % (n) | 69.3 (79) |
| Ulcerative findings | Cerebral infarction, % (n) | 24.6 (28) | |
| (+), % (n) | 5.3 (6) | Hemodialysis, % (n) | 3.5 (4) |
| (-) , % (n) | 94.7 (108) | Receiving antithrombotic agents | |
| Depth of invasion | Continue, % (n) | 100 (114) | |
| M/SM1, % (n) | 98.2 (112) | Discontinue, % (n) | 0 (0) |
| SM2-, % (n) | 1.8 (2) | Antithrombotic agents | |
| Pathological finding | Aspirin, % (n) | 43.9 (50) | |
| Differentiated type, % (n) | 95.6 (109) | Thienopyridine, % (n) | 10.5 (12) |
| Undifferentiated type, % (n) | 4.4 (5) | Cilostazol, % (n) | 5.3 (6) |
| lymphovascular infiltration | Warfarin, % (n) | 2.6 (3) | |
| (+), % (n) | 4.4 (5) | DOAC, % (n) | 20.2 (23) |
| (-) , % (n) | 95.6 (109) | Multiple antithrombotic agents, % (n) | 17.5 (20) |
As a result of the principle of ATT continuation, all patients continued to take antithrombotic agents during the perioperative period. These included aspirin (in 43.9% of the patients), thienopyridine (10.5%), cilostazol (5.3%), warfarin (2.6%), DOAC (20.2%), and multiple antithrombotic agents (17.5%). Comorbidities requiring ATT included cardiac disease in 69.3% (79/114) and cerebral infarction in 24.6% (28/114) of the patients. The rates of en-bloc, R0, and curative resection were 100%, 100%, and 90.4%, respectively. The median procedure time was 41.5 min (range 10-180). Endoscopic hemostasis was added to 68.8% (77/114) of SLE cases and 35.7% (40/114) of TLE cases.
The overall PDB rate was 7.9% (9/114) (90%CI: 4.7-13.1, P = 0.005), lower than 15%, indicating that the primary hypothesis of the present study was met. The E-PDB and L-PDB rates during the period of primary endpoint threshold calculation were 3.8% and 11.5%, respectively, based on our historical dataset. Sub-analysis showed that the E-PDB rate was 2.6% (3/114) (90%CI: 1.1-6.4, P = 0.51) and the L-PDB rate was 5.3% (6/114) (90%CI: 2.7-9.9, P < 0.0001), indicating that TLE significantly reduced L-PDB (Table 2).
| TLE group, n = 114 | 90%CI | P value | Threshold (%) | |
| Overall PDB, % (n) | 7.9 (9) | 4.7-13.1 | 0.005 | 15.3 |
| Early phase (E-PDB), % (n) | 2.6 (3) | 1.1-6.4 | 0.51 | 3.8 |
| Late phase (L-PDB), % (n) | 5.3 (6) | 2.7-9.9 | < 0.0001 | 11.5 |
We found significant differences between groups regarding tumor location (P = 0.005) and the continuation of antithrombotic agents (P < 0.0001) on the baseline characteristics of the TLE and control groups before propensity score matching (Table 3); therefore, we used propensity score matching to reduce these biases. Accordingly, the propensity score matching generated 58 matched pairs; baseline characteristics after matching are shown in Table 4. There were no significant differences in any factors between groups. A comparative analysis of PDB rates between groups is shown in Table 5. The TLE and control groups did not differ in the overall PDB [10.3% (6/58) vs 20.7% (12/58), P = 0.12] or E-PDB [5.2% (3/58) vs 3.5% (2/58), P = 1.00] rates. Conversely, there were significant differences in the L-PDB rate [5.2% (3/58) vs 17.2% (10/58), P = 0.04].
| TLE (n = 114) | Control (n = 132) | P value | |
| Age, mean ± SD (yr) | 75.5 ± 6.2 | 76.2 ± 6.5 | 0.37 |
| Sex | 0.68 | ||
| Male, % (n) | 86.0 (98) | 84.1 (111) | |
| Female, % (n) | 14.0 (16) | 15.9 (21) | |
| Location-1 | 0.51 | ||
| Upper, % (n) | 18.4 (21) | 15.2 (20) | |
| Middle, % (n) | 12.3 (14) | 13.6 (18) | |
| Lower, % (n) | 69.3 (79) | 71.2 (94) | |
| Location-2 | 0.005 | ||
| Anterior wall, % (n) | 8.8 (10) | 25.0 (33) | |
| Posterior wall, % (n) | 31.6 (36) | 19.7 (26) | |
| Lesser curvature, % (n) | 40.4 (46) | 37.9 (50) | |
| Greater curvature, % (n) | 19.3 (22) | 17.4 (23) | |
| Morphology | 0.53 | ||
| Protruded, % (n) | 43.0 (49) | 47.0 (62) | |
| Flat/depressed, % (n) | 57.0 (65) | 53.0 (70) | |
| Median tumor size (range) | 11.0 (2-70) | 12.0 (3-60) | 0.77 |
| Median specimen size (range) | 35.0 (18-95) | 35.0 (15-97) | 0.89 |
| Ulcerative findings | 0.79 | ||
| (+), % (n) | 5.3 (6) | 4.6 (6) | |
| (-), % (n) | 94.7 (108) | 95.5 (126) | |
| Depth of invasion | 0.09 | ||
| M/SM1, % (n) | 98.3 (112) | 93.9 (124) | |
| SM2-, % (n) | 1.8 (2) | 6.1 (8) | |
| Pathological finding | 0.07 | ||
| Differentiated type, % (n) | 95.6 (109) | 99.2 (131) | |
| Undifferentiated type, % (n) | 4.4 (5) | 0.8 (1) | |
| Lymphovascular infiltration | 0.74 | ||
| (+), % (n) | 4.4 (5) | 5.3 (7) | |
| (-), % (n) | 95.6 (109) | 94.7 (125) | |
| Median procedure time (range) | 41.5 (10-180) | 42.0 (7-215) | 0.84 |
| Receiving antithrombotic agents | < 0.0001 | ||
| Continue, % (n) | 100 (114) | 43.9 (58) | |
| Discontinue, % (n) | 0 (0) | 56.1 (74) |
| TLE (n = 58) | Control (n = 58) | P value | |
| Age, mean ± SD (yr) | 75.8 ± 6.4 | 77.7 ± 6.5 | 0.10 |
| Sex | 0.59 | ||
| Male, % (n) | 84.5 (49) | 87.9 (51) | |
| Female, % (n) | 15.5 (9) | 12.1 (7) | |
| Location-1 | 0.52 | ||
| Upper, % (n) | 19.0 (11) | 13.8 (8) | |
| Middle, % (n) | 6.9 (4) | 12.1 (7) | |
| Lower, % (n) | 74.1 (43) | 74.1 (43) | |
| Location-2 | 0.14 | ||
| Anterior wall, % (n) | 12.1 (7) | 24.1 (14) | |
| Posterior wall, % (n) | 34.5 (20) | 19.0 (11) | |
| Lesser curvature, % (n) | 34.5 (20) | 41.4 (24) | |
| Greater curvature, % (n) | 19.0 (11) | 15.5 (9) | |
| Morphology | 0.35 | ||
| Protruded, % (n) | 46.6 (27) | 55.2 (32) | |
| Flat/depressed, % (n) | 53.5 (31) | 44.8 (26) | |
| Median tumor size (range) | 9.5 (2-40) | 11.0 (3-43) | 0.54 |
| Median specimen size (range) | 34 (18-60) | 33 (17-78) | 0.71 |
| Ulcerative findings | 1.00 | ||
| (+), % (n) | 1.7 (1) | 1.7 (1) | |
| (-), % (n) | 98.3 (57) | 98.3 (57) | |
| Depth of invasion | 0.17 | ||
| M/SM1, % (n) | 98.3 (57) | 93.1 (54) | |
| SM2-, % (n) | 1.7 (1) | 6.9 (4) | |
| Pathological finding | - | ||
| Differentiated type, % (n) | 100 (58) | 100 (58) | |
| Undifferentiated type, % (n) | 0 (0) | 0 (0) | |
| Lymphovascular infiltration | 1.00 | ||
| (+), % (n) | 3.5 (2) | 3.5 (2) | |
| (-), % (n) | 96.6 (56) | 96.6 (56) | |
| Median procedure time (range) | 36.0 (10-78) | 37.0 (8-175) | 0.14 |
| Receiving antithrombotic agents | - | ||
| Continue, % (n) | 100 (58) | 100 (58) | |
| Discontinue, % (n) | 0 (0) | 0 (0) |
| TLE (n = 58) | Control (n = 58) | P value | |
| Overall PDB rate, % (n) | 10.3 (6) | 20.7 (12) | 0.12 |
| Early phase(E-PDB), % (n) | 5.2 (3) | 3.5 (2) | 1 |
| Late phase(L-PDB) , % (n) | 5.2 (3) | 17.2 (10) | 0.04 |
Table 6 shows the PDB rate per antithrombotic agent in the TLE and control groups. Due to the small number of registrations for each drug, no statistical analysis was performed. The overall PDB and L-PDB rates were relatively higher for DOAC and multiple antithrombotic agents’ treatment in the control group. The respective rates in the TLE group were lower than those in the control group for DOAC [13.0% (3/23) vs 23.1% (3/13) and 8.7% (2/23) vs 23.1% (3/13), respectively] and for multiple antithrombotic agents [10.0% (2/20) vs 32.4% (11/34) and 5.0% (1/20) vs 29.4% (10/34), respectively]. Regarding the E-PDB rate, there was a small difference between groups for these drugs.
| Overall | E-PDB | L-PDB | ||||
| TLE | Control | TLE | Control | TLE | Control | |
| Aspirin, % (n) | 6.0 (3/50) | 3.4 (2/59) | 2.0 (1/50) | 3.4 (2/59) | 4.0 (2/50) | 0 (0/59) |
| Thienopyridine, % (n) | 8.3 (1/12) | 9.1 (1/11) | 0 (0/12) | 0 (0/11) | 8.3 (1/12) | 9.1 (1/11) |
| Cilostazol, % (n) | 0 (0/6) | 0 (0/14) | 0 (0/6) | 0 (0/14) | 0 (0/6) | 0 (0/14) |
| Warfarin, % (n) | 0 (0/3) | 0 (0/1) | 0 (0/3) | 0 (0/1) | 0 (0/3) | 0 (0/1) |
| DOAC, % (n) | 13.0 (3/23) | 23.1 (3/13) | 4.3 (1/23) | 0 (0/13) | 8.7 (2/23) | 23.1 (3/13) |
| Multiple antithrombotic agents, % (n) | 10.0 (2/20) | 32.4 (11/34) | 5.0(1/20) | 2.9 (1/34) | 5.0 (1/20) | 29.4 (10/34) |
ESD for EGC is widely accepted as an effective treatment based on sufficient evidence. However, PDB remains a problem, especially in patients under ATT. We previously reported ATT as an independent risk factor for PDB, especially for L-PDB, in a retrospective cohort[5,6]. Additionally, there have been many reports on PDB in patients under ATT[7,18-24]. Two meta-analyses have reported the risk factors for PDB[8,25] and ATT was always included as a risk factor for PDB.
Several measures have been designed to counter this problem. The administration of rabeprazole or other proton pump inhibitor in perioperative days and the post-ESD method of prophylactic hemostatic coagulation of visible ulcer vessels are widely used[26,27]. Conversely, regarding SLE, the SAFE trial in Japan suggested that it may not contribute to PDB prevention[28]; however, this evidence-based, randomized study did not include patients under ATT. Therefore, the effectiveness of SLE has not yet been fully evaluated in these patients. According to our previous reports[5,6], ATT was an independent risk factor for PDB, even though all patients underwent SLE. Although these retrospective cohorts were not analyzed for the efficacy of SLE, ATT was not found to be associated with E-PDB, whereas it was significantly associated with L-PDB. Therefore, considering that L-PDB can be reduced by performing interventional endoscopy at a later period, we focused on the effectiveness of TLE, a simple and preferred method of prevention which is easily accepted by every endoscopist.
Regarding our primary endpoint, the overall PDB rate was significantly lower than the threshold that we assumed based on our historical dataset. Therefore, our hypothesis was met, showing that TLE reduces the overall PDB rate and is useful for preventing bleeding in patients receiving ATT. Thus, TLE seems to be more effective against L-PDB. Several previous retrospective studies have reported a PDB rate of 11.1%-23.7% for patients under ATT[7,18-23]. Furthermore, a prospective observational study by Ono et al[24] showed that PDB occurrence in these patients was as high as 26.1%. The overall PDB rate in the present study is lower than previously reported. To date, two single-arm retrospective studies have been published on TLE: One reporting a rate of 11.1% for PDB in patients under ATT undergoing TLE[18] and the other investigating the efficacy of TLE showing that the PDB rate could be reduced to 2.6%[29]; however, ATT was administered only in 18% of the cases, which might explain why PDB occurrence was so low.
Regarding our secondary endpoint, the overall PDB rate in the TLE group was lower than that in the control group (10.3% vs 20.7%); however, the difference was not significant, despite being as high as 10% (P = 0.12). This might be due to the small number of cases analyzed (58 per group) based on propensity matching. As all registered patients in the present study continued ATT, the 114 samples were reduced to about half. Conversely, the L-PDB rate was significantly lower in the TLE group than in the historical control group (5.2% vs 17.2%, P = 0.04). We speculate that the usefulness of TLE is associated with the healing process of artificial ulcers. The bottom of the ulcer is covered with slough within a few days, and the regenerating epithelium, including granulation tissue, gradually develops from the margin and bottom of the ulcer. Also, neovascularization from submucosal vessels is observed in this period[30]. Takeuchi et al[30] described the ulcer healing process using a rat model, showing that neovascularization starts from the margin and bottom of the ulcer at around 5 d. Therefore, bleeding in gastric ESD may be due to two types of blood vessels: vessels that have been coagulated at the time of ESD and re-bleed causing E-PDB and newly-grown vessels from the submucosal layer that cause L-PDB. When we performed TLE around POD 5, it was possible to perform prophylactic hemostasis against neovascular vessels, which explains why we could reduce PDB, and especially L-PDB, more effectively.
To the best of our knowledge, no other prospective study has reported that PDB can be effectively prevented despite continuing ATT. The advantage of this study is not only its prospective nature but that all patients continued ATT. There were several reasons why we prioritized the continuation of antithrombotic agents. First, the direct effect of withdrawing antithrombotics E-PDB and not L-PDB-induced. In our previous reports, ATT was discontinued 2-7 d before ESD, as per current guidelines. Moreover, warfarin was a significant factor only in E-PDB[6]. Warfarin is basically given to patients at high risk for thrombosis, and HBT not only has poor evidence of thromboprophylaxis but also promotes PDB; thus, all agents, including warfarin, were continued as long as possible. Moreover, regarding the timing of drug resumption, we previously set POD 2 at the time of meal onset, similar to previous reports. Most antithrombotics are likely to promote bleeding after resumption[5,6]. Therefore, drug continuation and prevention of L-PDB with TLE are ideal. In their retrospective study, Igarashi et al[19] also recommended ATT continuation which did not show a significant increase in PDB compared to controls. Second, even for patients at low risk of thromboembolism, withdrawal of antithrombotics always increases the risk of a thromboembolic event[31,32]; therefore, these agents should be withdrawn only if they can be safely discontinued since the outcome is poor once a thromboembolic event has occurred. Maulaz et al[31] reported that patients with cerebral infarction tend to relapse within about 10 d after aspiring withdrawal; the same was reported by Giuseppe et al[32] for patients with ischemic heart disease. Conversely, Kazi et al[33] reported that bleeding in the patients with ischemic heart disease increases mortality due to cardiac heart failure. Although it is difficult to balance bleeding and thromboembolic events since both have to be prevented in such patients, more robust intervention in PDB is required to avoid thromboembolic events as much as possible. The guidelines recommend drug withdrawal or change, or postponement of treatment depending on the risk; however, timely treatment cannot be postponed in a patient with cancer, and changing drugs also carries the risk of thrombosis. Hence, we consider that our findings are meaningful and may contribute to the safety of ESD in patients receiving antithrombotics, even with drug continuation.
We also calculated the PDB rate according to the antithrombotic agents used in the TLE and control groups. As the sample size was insufficient for statistical analysis, we only calculated the PDB ratio by antithrombotic agent. DOAC and multiple agents seemed to lead to higher bleeding rates than other agents, overall and in the late phase. Connolly et al[34] performed a prospective study to compare the efficacy of dabigatran and warfarin in preventing thromboembolism and showed that dabigatran reduces intracranial bleeding but significantly increases gastrointestinal bleeding compared to warfarin. Regarding multiple agents, Halas et al[35] reported that they increase the risk of gastrointestinal bleeding compared to single agents and that this risk is 7.4 times higher among patients taking aspirin and clopidogrel than in those receiving non-antithrombotic agents. Although the subjects in these reports differ from scheduled ESD cases, these drugs require particular attention considering PDB. Our L-PDB rate seems to be the best reported so far, especially for DOAC or DAPT/multiple agents, although it is necessary to study larger patient cohorts receiving these agents.
This study had two limitations; first, it was conducted in a single facility; and second, it was a single-arm and not a randomized controlled trial.
In conclusion, this TLE addition in gastric ESD reduced the overall PDB rate and was particularly effective in preventing L-PDB in patients receiving antithrombotic agents, including DOAC and DAPT, which significantly increase the risk of PDB. TLE was effective even after continuous ATT. Multicenter randomized control trials are required to verify the effectiveness of TLE.
Endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) is minimally invasive and the gold standard for treatment for endoscopic resection. However, the problem of postoperative delayed bleeding (PDB) as a major adverse event remains.
The PDB rate under antithrombotic therapy (ATT) is higher than non-ATT and the bleeding period tends to be a late phase. Despite several attempts against PDB have been reported, there are no effective preventive methods yet.
We attempted to verify the efficacy of third-look endoscopy (TLE) against PDB in patients under ATT.
This is a prospective study in a single center. We enrolled patients with EGC receiving ATT, and TLE was added to conventional ESD, including second-look endoscopy. Additionally, we compared the PDB rate with that of a historical control group subjected to conventional ESD under ATT, using propensity score matching.
The PDB rate of patients adding TLE was lower than the threshold which we set, and it was significantly lower, especially late-onset PDB (L-PDB). Regarding the comparison with the historical control group, the L-PDB rate in the TLE group was lower.
TLE is a simple method that reduces the overall PDB, especially L-PDB, in patients under ATT and is widely acceptable by endoscopists.
This study is not a randomized controlled trial (RCT); therefore, we consider it necessary to investigate RCT against a larger patient sample.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira CN S-Editor: Zhang H L-Editor: A P-Editor: Ma YJ
| 1. | Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H; Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 2. | Vital Statistics Japan. In: Ministry of Health, Labour and Welfare. Available from: https://www.mhlw.go.jp/english/. [Cited in This Article: ] |
| 3. | Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Nunobe S, Kakeji Y, Nashimoto A; Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21:144-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 289] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 4. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 345] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A, Taguri M, Morita S, Maeda S, Tanaka K. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Sato C, Hirasawa K, Koh R, Ikeda R, Fukuchi T, Kobayashi R, Kaneko H, Makazu M, Maeda S. Postoperative bleeding in patients on antithrombotic therapy after gastric endoscopic submucosal dissection. World J Gastroenterol. 2017;23:5557-5566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Furuhata T, Kaise M, Hoteya S, Iizuka T, Yamada A, Nomura K, Kuribayashi Y, Kikuchi D, Matsui A, Ogawa O, Yamashta S, Mitani T. Postoperative bleeding after gastric endoscopic submucosal dissection in patients receiving antithrombotic therapy. Gastric Cancer. 2017;20:207-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Libânio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc. 2016;84:572-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, Aizawa Y, Yamashita T, Atarashi H, Horie M, Ohe T, Doi Y, Shimizu A, Chishaki A, Saikawa T, Yano K, Kitabatake A, Mitamura H, Kodama I, Kamakura S. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Kataoka Y, Tsuji Y, Hirasawa K, Takimoto K, Wada T, Mochizuki S, Ohata K, Sakaguchi Y, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Endoscopic tissue shielding to prevent bleeding after endoscopic submucosal dissection: a prospective multicenter randomized controlled trial. Endoscopy. 2019;51:619-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Tsuji Y, Fujishiro M, Kodashima S, Ono S, Niimi K, Mochizuki S, Asada-Hirayama I, Matsuda R, Minatsuki C, Nakayama C, Takahashi Y, Sakaguchi Y, Yamamichi N, Koike K. Polyglycolic acid sheets and fibrin glue decrease the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms (with video). Gastrointest Endosc. 2015;81:906-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Kawata N, Ono H, Takizawa K, Kakushima N, Tanaka M, Igarashi K, Yoshida M, Kishida Y, Iwai T, Ito S, Imai K, Hotta K, Ishiwatari H, Matsubayashi H. Efficacy of polyglycolic acid sheets and fibrin glue for prevention of bleeding after gastric endoscopic submucosal dissection in patients under continued antithrombotic agents. Gastric Cancer. 2018;21:696-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1575] [Cited by in F6Publishing: 1810] [Article Influence: 258.6] [Reference Citation Analysis (0)] |
| 14. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 15. | Fujimoto K, Fujishiro M, Kato M, Higuchi K, Iwakiri R, Sakamoto C, Uchiyama S, Kashiwagi A, Ogawa H, Murakami K, Mine T, Yoshino J, Kinoshita Y, Ichinose M, Matsui T; Japan Gastroenterological Endoscopy Society. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 16. | Kato M, Uedo N, Hokimoto S, Ieko M, Higuchi K, Murakami K, Fujimoto K. Guidelines for Gastroenterological Endoscopy in Patients Undergoing Antithrombotic Treatment: 2017 Appendix on Anticoagulants Including Direct Oral Anticoagulants. Dig Endosc. 2018;30:433-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 17. | Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, Schulman S, Turpie AG, Hasselblad V, Ortel TL; BRIDGE Investigators. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373:823-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 695] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 18. | Gotoda T, Hori K, Iwamuro M, Kono Y, Miura K, Kanzaki H, Kawano S, Kawahara Y, Okada H. Evaluation of the bleeding risk with various antithrombotic therapies after gastric endoscopic submucosal dissection. Endosc Int Open. 2017;5:E653-E662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Igarashi K, Takizawa K, Kakushima N, Tanaka M, Kawata N, Yoshida M, Ito S, Imai K, Hotta K, Ishiwatari H, Matsubayashi H, Ono H. Should antithrombotic therapy be stopped in patients undergoing gastric endoscopic submucosal dissection? Surg Endosc. 2017;31:1746-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Sanomura Y, Oka S, Tanaka S, Yorita N, Kuroki K, Kurihara M, Mizumoto T, Yoshifuku Y, Chayama K. Taking Warfarin with Heparin Replacement and Direct Oral Anticoagulant Is a Risk Factor for Bleeding after Endoscopic Submucosal Dissection for Early Gastric Cancer. Digestion. 2018;97:240-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Takeuchi T, Ota K, Harada S, Edogawa S, Kojima Y, Tokioka S, Umegaki E, Higuchi K. The postoperative bleeding rate and its risk factors in patients on antithrombotic therapy who undergo gastric endoscopic submucosal dissection. BMC Gastroenterol. 2013;13:136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Tounou S, Morita Y, Hosono T. Continuous aspirin use does not increase post-endoscopic dissection bleeding risk for gastric neoplasms in patients on antiplatelet therapy. Endosc Int Open. 2015;3:E31-E38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Yoshio T, Tomida H, Iwasaki R, Horiuchi Y, Omae M, Ishiyama A, Hirasawa T, Yamamoto Y, Tsuchida T, Fujisaki J, Yamada T, Mita E, Ninomiya T, Michitaka K, Igarashi M. Effect of direct oral anticoagulants on the risk of delayed bleeding after gastric endoscopic submucosal dissection. Dig Endosc. 2017;29:686-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Ono S, Fujishiro M, Yoshida N, Doyama H, Kamoshida T, Hirai S, Kishihara T, Yamamoto Y, Sakae H, Imagawa A, Hirano M, Koike K. Thienopyridine derivatives as risk factors for bleeding following high risk endoscopic treatments: Safe Treatment on Antiplatelets (STRAP) study. Endoscopy. 2015;47:632-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Dong J, Wei K, Deng J, Zhou X, Huang X, Deng M, Lü M. Effects of antithrombotic therapy on bleeding after endoscopic submucosal dissection. Gastrointest Endosc. 2017;86:807-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C, Yamamoto S, Higashino K, Iishi H, Tatsuta M. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 28. | Mochizuki S, Uedo N, Oda I, Kaneko K, Yamamoto Y, Yamashina T, Suzuki H, Kodashima S, Yano T, Yamamichi N, Goto O, Shimamoto T, Fujishiro M, Koike K; SAFE Trial Study Group. Scheduled second-look endoscopy is not recommended after endoscopic submucosal dissection for gastric neoplasms (the SAFE trial): a multicentre prospective randomised controlled non-inferiority trial. Gut. 2015;64:397-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Tano S, Horiki N, Omata F, Tanaka K, Hamada Y, Katsurahara M, Ninomiya K, Nishikawa K, Nojiri K, Yamada R, Inoue H, Gabazza EC, Katayama N, Takei Y. Second and third-look endoscopy for the prevention of post-ESD bleeding. Medicine (Baltimore). 2015;94:e491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Takeuchi K, Kishi S. [Studies on the fine vessels in the healing process of acetic acid ulcer in the rat stomach--scanning electron microscopic studies using plastic vascular models]. Nihon Shokakibyo Gakkai Zasshi. 1983;80:9-15. [PubMed] [Cited in This Article: ] |
| 31. | Maulaz AB, Bezerra DC, Michel P, Bogousslavsky J. Effect of discontinuing aspirin therapy on the risk of brain ischemic stroke. Arch Neurol. 2005;62:1217-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 32. | Biondi-Zoccai GG, Lotrionte M, Agostoni P, Abbate A, Fusaro M, Burzotta F, Testa L, Sheiban I, Sangiorgi G. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27:2667-2674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 499] [Cited by in F6Publishing: 442] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 33. | Kazi DS, Leong TK, Chang TI, Solomon MD, Hlatky MA, Go AS. Association of spontaneous bleeding and myocardial infarction with long-term mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2015;65:1411-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7917] [Cited by in F6Publishing: 7742] [Article Influence: 516.1] [Reference Citation Analysis (0)] |
| 35. | Hallas J, Dall M, Andries A, Andersen BS, Aalykke C, Hansen JM, Andersen M, Lassen AT. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. BMJ. 2006;333:726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 267] [Article Influence: 14.8] [Reference Citation Analysis (0)] |