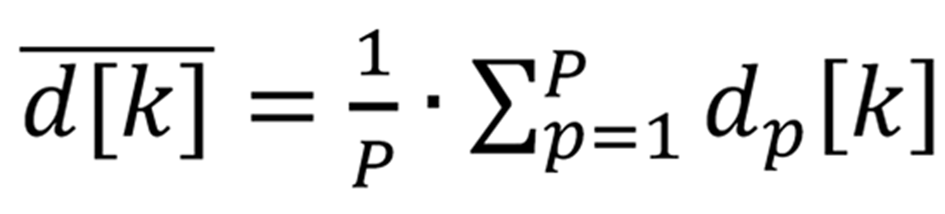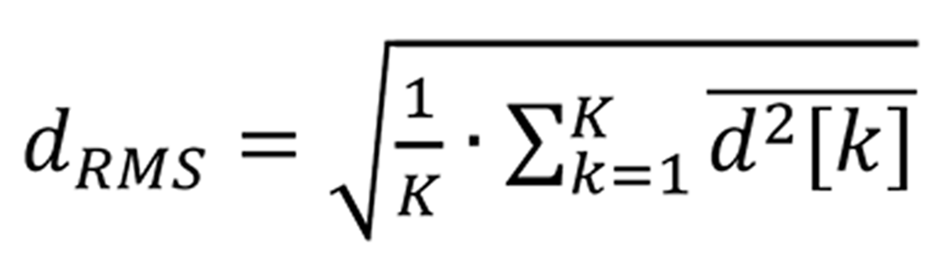Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5836
Peer-review started: May 21, 2020
First decision: May 29, 2020
Revised: June 11, 2020
Accepted: September 17, 2020
Article in press: September 17, 2020
Published online: October 14, 2020
Degree of portal hypertension (PH) is the most important prognostic factor for the decompensation of liver cirrhosis and death, therefore adequate care for patients with liver cirrhosis requires timely detection and evaluation of the presence of clinically significant PH (CSPH) and severe PH (SPH). As the most accurate method for the assessment of PH is an invasive direct measurement of hepatic venous pressure gradient (HVPG), the search for non-invasive methods to diagnose these conditions is actively ongoing.
To evaluate the feasibility of parameters of endogenously induced displacements and strain of liver to assess degree of PH.
Of 36 patients with liver cirrhosis and measured HVPG were included in the case-control study. Endogenous motion of the liver was characterized by derived parameters of region average tissue displacement signal (dantero, dretro, dRMS) and results of endogenous tissue strain imaging using specific radiofrequency signal processing algorithm. Average endogenous strain µ and standard deviation σ of strain were assessed in the regions of interest (ROI) (1 cm × 1 cm and 2 cm × 2 cm in size) and different frequency subbands of endogenous motion (0-10 Hz and 10-20 Hz).
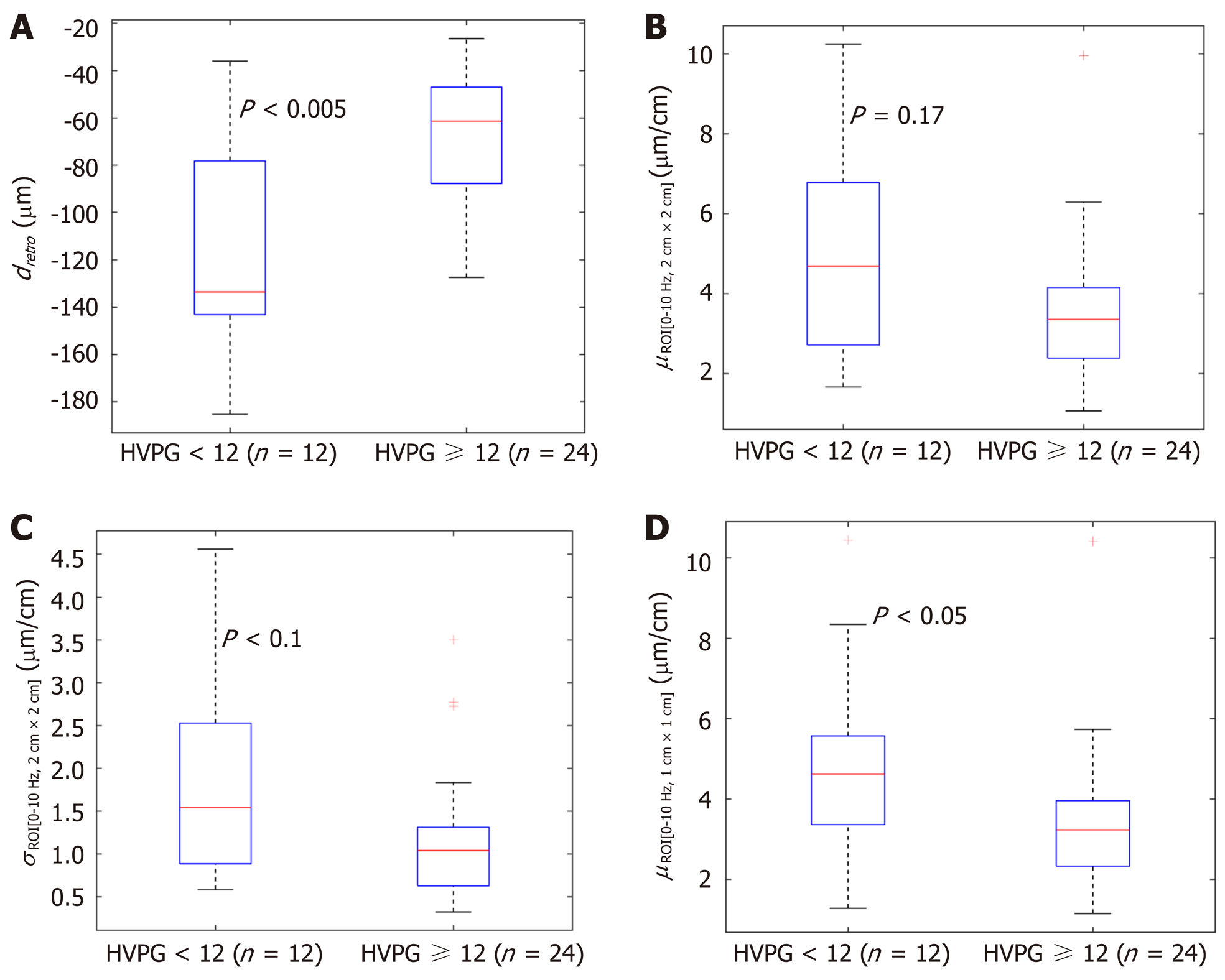
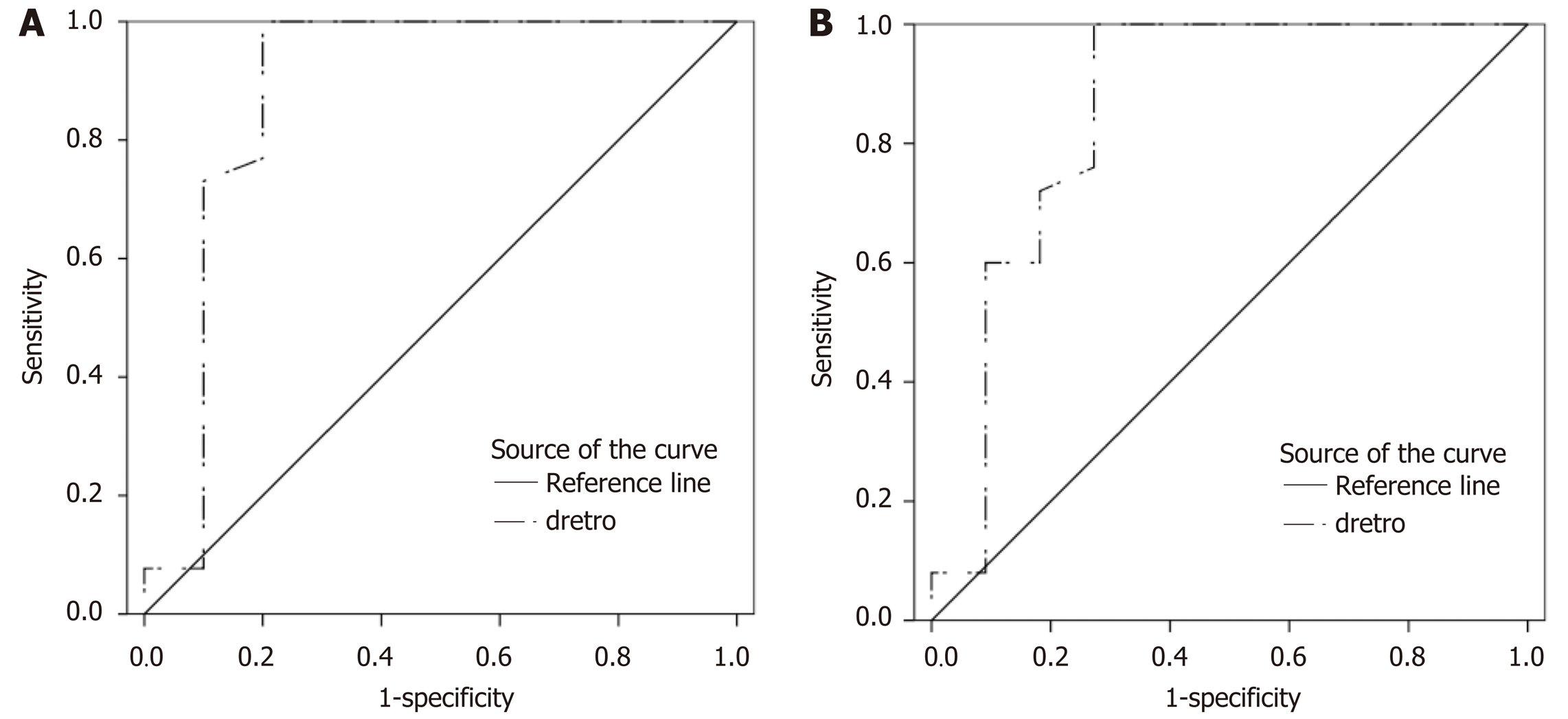
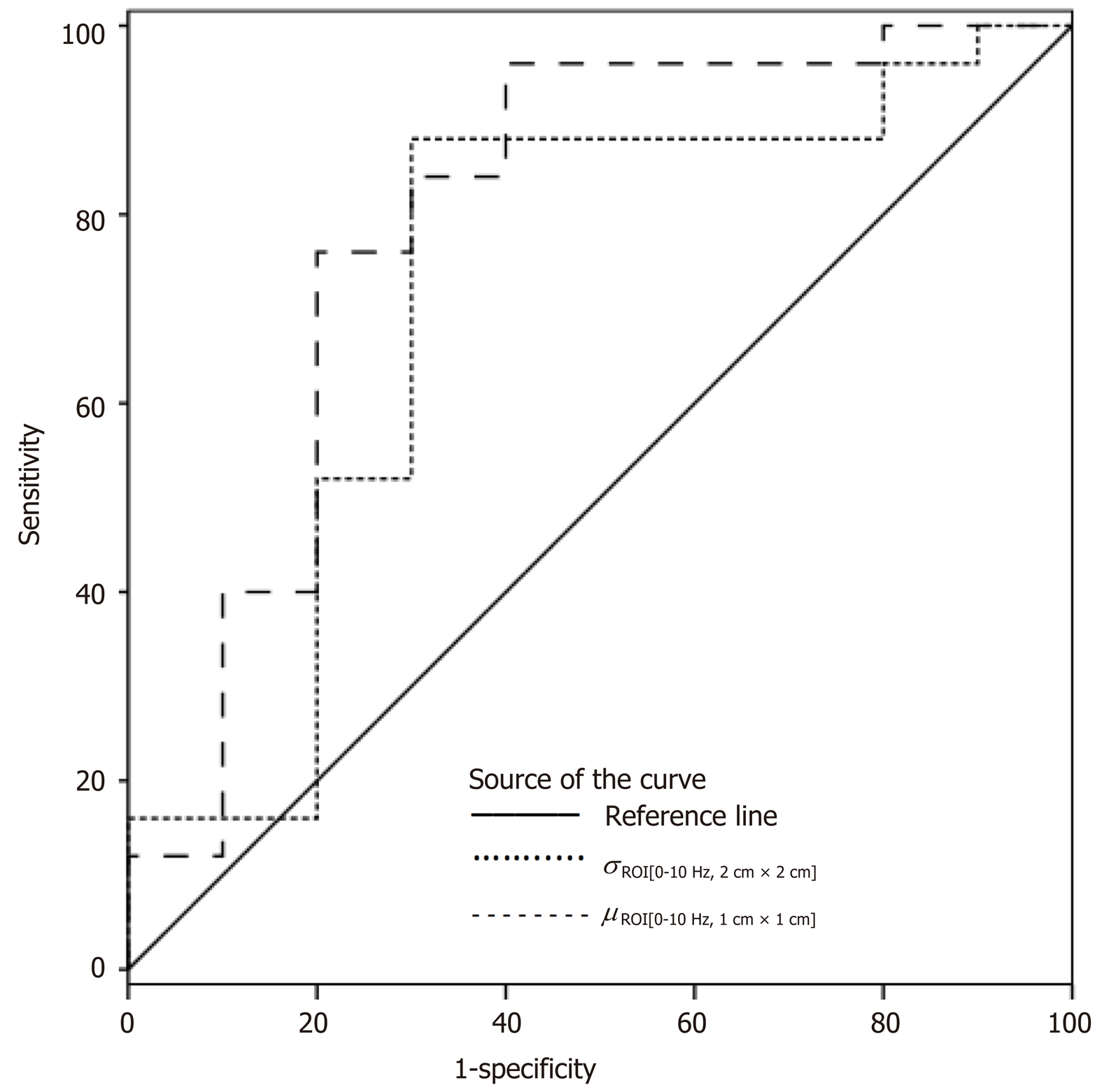
Four parameters showed statistically significant (P < 0.05) correlation with HVPG measurement. The strongest correlation was obtained for the standard deviation of strain (estimated at 0-10 Hz and 2 cm × 2 cm ROI size). Three parameters showed statistically significant differences between patient groups with CSPH, but only dretro showed significant results in SPH analysis. According to ROC analysis area under the curve (AUC) of the σROI[0…10Hz, 2 cm × 2 cm] parameter reached 0.71 (P = 0.036) for the diagnosis of CSPH; with a cut-off value of 1.28 μm/cm providing 73% sensitivity and 70% specificity. AUC for the diagnosis of CSPH for µROI[0…10Hz, 1 cm × 1 cm] was 0.78 (P = 0.0024); with a cut-off value of 3.92 μm/cm providing 73% sensitivity and 80% specificity. Dretro parameter had an AUC of 0.86 (P = 0.0001) for the diagnosis of CSPH and 0.84 (P = 0.0001) for the diagnosis of SPH. A cut-off value of -132.34 μm yielded 100% sensitivity for both conditions, whereas specificity was 80% and 72% for CSPH and SPH respectively.
The parameters of endogenously induced displacements and strain of the liver correlated with HVPG and might be used for non-invasive diagnosis of PH.
Core Tip: In this study, we aimed to evaluate the feasibility of parameters of endogenously induced displacements and strain of the liver to assess the degree of portal hypertension. Endogenous motion of the liver was characterized by derived parameters of region average tissue displacement signal and results of endogenous tissue strain imaging using specific radiofrequency signal processing algorithm. Our proposed parameters correlated with hepatic venous pressure gradient and statistically significant differences were observed between patient groups with clinically significant and severe portal hypertension. To our knowledge it is the first study to evaluate prognostic potential of endogenous motion parameters to detect clinically significant and severe portal hypertension.
- Citation: Gelman S, Sakalauskas A, Zykus R, Pranculis A, Jurkonis R, Kuliavienė I, Lukoševičius A, Kupčinskas L, Kupčinskas J. Endogenous motion of liver correlates to the severity of portal hypertension. World J Gastroenterol 2020; 26(38): 5836-5848
- URL: https://www.wjgnet.com/1007-9327/full/v26/i38/5836.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i38.5836
The burden of chronic liver diseases is growing and decompensated liver cirrhosis is responsible for a million of deaths per year worldwide[1]. The grade of portal hypertension (PH) expressed by hepatic venous pressure gradient (HVPG) is an important prognostic factor for the decompensation of liver cirrhosis and formation of life threatening complications, such as ascites, gastroesophageal varices and hepatic encephalopathy[2,3]. HVPG value greater than 5 mmHg is considered to be PH. Clinically significant PH (CSPH) is diagnosed when HVPG values exceed 10 mmHg and is associated with the formation of gastroesophageal varices, ascites and hepatorenal syndrome. When HVPG values exceed 12 mmHg severe PH (SPH) is diagnosed with higher risk of variceal bleeding and decompensation. Thus the evaluation of the presence of CSPH and SPH are of utmost importance[4,5].
Direct measurement of HVPG is regarded as the most accurate method for the assessment of PH, however this procedure is currently reserved for specialized centers, as it is invasive, costly and requires expertise[2,6].
Ultrasound elastography is one of the most widely used non-invasive alternatives for the diagnosis of PH. The method is appealing due to its low cost, applicability and availability[4,7]. Several ultrasound elastography techniques have been developed: Strain imaging [strain elastography (SE), using internal/external compression stimuli, or acoustic radiation force impulse] and shear wave imaging [point shear wave elastography (pSWE), 2D-SWE and transient elastography (TE)][8,9]. Out of existing ultrasound elastography techniques TE, using Fibroscan (Echosens, France), has shown most promising results in diagnosing CSPH with area under the receiver operating characteristic curve (AUROC) of 0.90, sensitivity 87% and specificity 85%, whereas other shear wave techniques showed lower sensitivity in this field[10-12]. The readings of TE, pSWE and 2D-SWE are affected by active inflammation, cholestasis, fatty liver and biliary obstruction thus TE performance is not optimal in patients with obesity, ascites, narrow intercostal space. These limitations encourage the search for another alternative technique that could be applied in this diverse patient population.
SE is an ultrasound elastography technique widely used for the examination of musculoskeletal system[13], breast and thyroid pathologies[14], however it has been scarcely evaluated in the setting of liver cirrhosis and PH. SE measures axial displacement of tissue caused by manual compression or physiological shifts inside the body (cardiovascular pulsatility or breathing)[15,16]. Elasticity is displayed as color coded elastograms: Areas with lower strain are displayed in blue and areas with higher strain in red[11,15]. The drawback of this technique is that it measures the strain of tissues and the resulting elastogram is not quantifiable[16]. To obtain more accurate and reproducible results various semi-quantitative methods have been developed[15]. Different scores for semi-quantitative interpretation of SE have been proposed: The German Elasticity Score, the Japanese Elasticity Score, the LF Index, calculation of strain[9,17]. Other research groups develop and apply specific algorithms for quantitative evaluation of elasticity using a number of statistical parameters, derived from the distribution of recorded strains within a region of interest (ROI). The key parameters are: Mean strain, standard deviation of the mean; the percentage of blue area; complexity of the blue areas[18-23]. One advantage of this technique is the ability to evaluate the inhomogeneity of strain in larger areas of the liver[24,25]. Also, what is more important, SE readings are not affected by hepatic inflammation, jaundice, liver congestion, fatty degeneration, obesity, ascites or narrow intercostal spaces as is the case with other elastography modalities[15,26-28].
Studies concerning SE performance in PH are scarce. Ochi et al[29] reported that SE was useful for the diagnosis of PH in patients with non-alcoholic liver disease. Hirooka et al[30] evaluated SE performance in CSPH and SPH using elastic ratio. In their study AUROC for the diagnosis of CSPH was 0.83 and for the diagnosis of SPH – 0.78.
Our group has applied SE technique to assess the strain of liver tissue caused by endogenous motion of the beating heart and developed a specific radiofrequency (RF) signal analysis algorithm to calculate the parameters for quantification of strain in liver tissue. We have previously applied this RF ultrasound-based tissue strain imaging method to characterize tissue elasticity in vitro[31] and demonstrated the feasibility of this method in diagnosing liver fibrosis in patients with hepatitis C virus[32]. The aim of present study was to evaluate the ability and feasibility of endogenously induced displacements and strain on the liver to assess the degree of PH, using this specifically developed RF signal analysis algorithm.
Patients with hepatitis C virus and/or alcohol induced liver cirrhosis, hospitalized for the HVPG measurement procedure to the Hospital of Lithuanian University of Health Sciences, Department of Gastroenterology were included in this study. Exclusion criteria were: Pre- or post-hepatic causes of PH, cardiovascular disease, current use of beta-blockers or other vasoactive drugs, presence of ascites. Demographic data, medical history, aethiology of liver disease and HVPG values were recorded. The diagnosis of liver cirrhosis was based on clinical, laboratory and radiologic data and/or histology.
The study was approved by Kaunas Region Biomedical Research Ethics Committee (2015-08-24, No. BE-2-28, Kaunas, Lithuania) and a written informed consent to participate in the study was acquired from all participants.
We collected data using ultrasound scanner Ultrasonix Sonix Touch (Analogic Ultrasound, Canada), which allows to collect raw RF signals of all scanning lines. The ultrasound scanning was performed on the day of HVPG measurement prior to the procedure. Scanning protocol and the main RF data acquisition parameters were described in previous paper[32].
Invasive HVPG measurement procedure was used to assess the degree of PH. The procedure was performed according to standard as described by previous authors[33]. Portal pressure was considered to be normal for HVPG values of 1-5 mmHg. When HVPG values exceeded 6 mmHg, PH was diagnosed. HVPG values of ≥ 10 mmHg represented clinically significant and ≥ 12 mmHg – SPH.
Endogenous motion of the liver was characterized by derived parameters of region average tissue displacement signal and results of endogenous tissue strain imaging.
The displacements along the scanning beam line were estimated applying cross-correlation based technique. The signals were interpolated before calculation of cross-correlation. Length of the correlation window was set to 6 wavelengths and the overlap was 50%. The detailed description of the method could be found in previous publication[32]. The sequences of displacement images were obtained as a result of the displacement estimation stage.
The user predefined regions of interest (ROI) in all images of displacements were averaged in space, thus obtaining the region average liver tissue displacement signal. The average displacement signal could be expressed as follows:
Formula 1 where p = 1…P, P – number of displacement data points in a ROI of a subsector, dp[k] – displacement signal at p-th spatial position in a ROI, k = 1…K, K – acquisition instance (frame number).
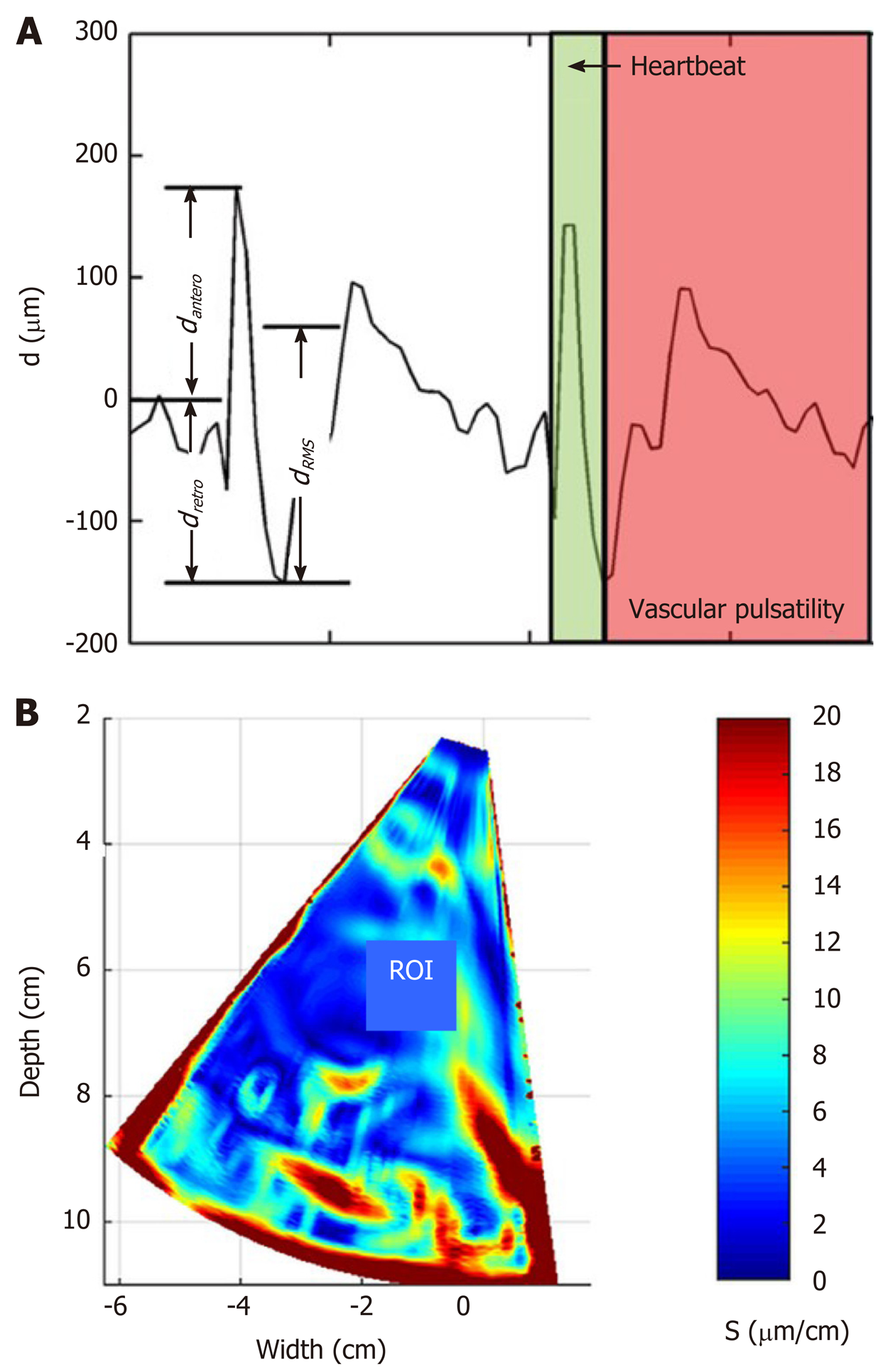
Afterwards, the motion of the liver was characterized by three parameters of the region average tissue displacement signal (assessment example is presented in Figure 1A): (1) Maximal amplitude of motion towards the ultrasonic probe dantero; (2) Maximal amplitude of motion backwards from the ultrasonic probe dretro; and (3) Average level of motion by root-mean-square dRMS value:
Formula 2 where k = 1…K, K – acquisition instance (frame number).
The endogenous strain map was derived from the temporal sequence of the displacement images. The strain maps were obtained taking the gradient of integrated spectral coefficient. An example of derived strain map is presented in Figure 1B. The description of data processing methods used for parametric imaging of endogenous strain was presented in detail in previous study[32].
The local quantitative assessment of the strain was performed by analyzing the distribution of derived strain map values in user predefined rectangular ROI of constant size (see assessment example in Figure 1B). The investigation was performed in 2 frequency subbands (integration ranges for lower f1 and f2 frequencies 0-10 Hz and higher 10-20 Hz, respectively) and 2 sizes of ROI (1 cm × 1 cm and 2 cm × 2 cm). The ROI selection criteria were based on visual evaluation of B scans and the obtained endogenous strain maps. At first, the sequences of B mode images were carefully revised with the aim to identify appropriate regions. The regions that contained only liver parenchyma without clearly visible blood vessels in B scans were outlined by the observer in the spatially corresponding images of endogenous strain.
All 11 investigated parameters of endogenous motion of liver together with a description are provided in Table 1.
| No. | Parameter | Description |
| 1 | dantero | Maximal amplitude of endogenous displacements towards the probe, μm |
| 2 | dretro | Maximal amplitude of the displacements backward, μm |
| 3 | dRMS | Average level of motion, μm |
| 4 | µROI[0…10 Hz, 2 cm × 2 cm] | Average strain [estimated for the 0…10 Hz sub-band of endogenous motion in the 2 cm × 2 cm ROI], μm/cm |
| 5 | σROI[0…10 Hz, 2 cm × 2 cm] | Standard deviation of strain [0…10 Hz, 2 cm × 2 cm], μm/cm |
| 6 | µROI[0…10 Hz, 1 cm × 1 cm] | Average strain [0…10 Hz, 1 cm × 1 cm], μm/cm |
| 7 | σROI[0…10 Hz, 1 cm × 1 cm] | Standard deviation of strain [0…10 Hz, 1 cm × 1 cm], μm/cm |
| 8 | µROI[10…20 Hz, 2 cm × 2 cm] | Average strain [10…20 Hz, 2 cm × 2 cm], μm/cm |
| 9 | σROI[10…20 Hz, 2 cm × 2 cm] | Standard deviation of strain [10…20 Hz, 2 cm × 2 cm], μm/cm |
| 10 | µROI[10…20 Hz, 1 cm × 1 cm] | Average strain [10…20 Hz, 1 cm × 1 cm], μm/cm |
| 11 | σROI[10…20 Hz, 1 cm × 1 cm] | Standard deviation of strain [10…20 Hz, 1 cm × 1 cm], μm/cm |
Statistical analysis was performed using SPSS 25.0 and Medcalc softwares. Kolmogorov–Smirnov test was used to check data normality. Descriptive statistics are provided as median and range for non-parametric data. Patients were divided into groups according to HVPG values: (1) Patients without CSPH (HVPG < 10 mmHg); (2) Patients with CSPH (HVPG ≥ 10 mmHg); (3) Patients without SPH (HVPG < 12 mmHg); and (4) Patients with SPH (HVPG ≥ 12 mmHg). Differences between the groups were evaluated using the Mann-Whitney’s test. Correlations were performed using Spearman’s correlation and expressed by Spearman’s coefficient. ROC curves were created to assess the predictive values of RF parameters for the degree of PH. AUC, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated. The cut-off value according to Youden’s index was chosen for the analysis of RF parameters. Statistical significance was established at P < 0.05.
Of 36 patients were included in the study. Mean age (± SD) of the subjects was 54.25 ± 8.82; 58.3% were male. Hepatitis C induced liver cirrhosis was diagnosed in 63.9% of cases. The summary of the demographic data is presented in Table 2.
| Variable | Characteristics (n = 36) |
| Sex (male/female; %) | 58.3/41.7 |
| Age (yr; SD) | 54.25 (8.82) |
| Aethiology (% of patients) | |
| Alcohol cirrhosis | 36.1 |
| HCV cirrhosis | 63.9 |
| Child-Pugh score (A/B/C; % of patients) | 58.3/36.2/5.6 |
| HVPG (mmHg; SD) | 14.3 (5.9) |
| HVPG 1-5 mmHg (% of patients) | 2.8 |
| HVPG 6-9 mmHg (% of patients) | 25 |
| CSPH; HVPG ≥ 10 mmHg (% of patients) | 72.2 |
| SPH; HVPG ≥ 12 mmHg (% of patients) | 69.4 |
We determined the correlation between the investigated strain parameters and invasive HPVG measurement. Spearman’s correlation coefficient (ρ) values and P value for the assessment of statistical significance are presented in Table 3. Four parameters showed statistically significant (P < 0.05) correlation with HVPG measurement. The strongest correlation was obtained for the standard deviation of strain (estimated at 0-10 Hz integration range and 2 cm × 2 cm ROI size). The directions of correlations met the expectation in almost all the cases. Negative correlation confirmed that the derived strain estimates decreased with an increment of HVPG.
| No. | Parameter | Spearman’s ρ | P value |
| 1 | dantero | -0.31 | 0.07 |
| 2 | dretro | 0.34 | 0.04 |
| 3 | dRMS | -0.33 | 0.05 |
| 4 | µROI[0…10 Hz, 2 × 2 cm] | -0.38 | 0.04 |
| 5 | σROI[0…10 Hz, 2 cm × 2 cm] | -0.42 | 0.01 |
| 6 | µROI[0…10 Hz, 1 cm × 1 cm] | -0.38 | 0.02 |
| 7 | σROI[0…10 Hz, 1 cm × 1 cm] | -0.27 | 0.11 |
| 8 | µROI[10…20 Hz, 2 cm × 2 cm] | -0.19 | 0.28 |
| 9 | σROI[10…20 Hz, 2 cm × 2 cm] | -0.14 | 0.43 |
| 10 | µROI[10…20 Hz, 1 cm × 1 cm] | -0.16 | 0.34 |
| 11 | σROI[10…20 Hz, 1 cm × 1 cm] | -0.16 | 0.36 |
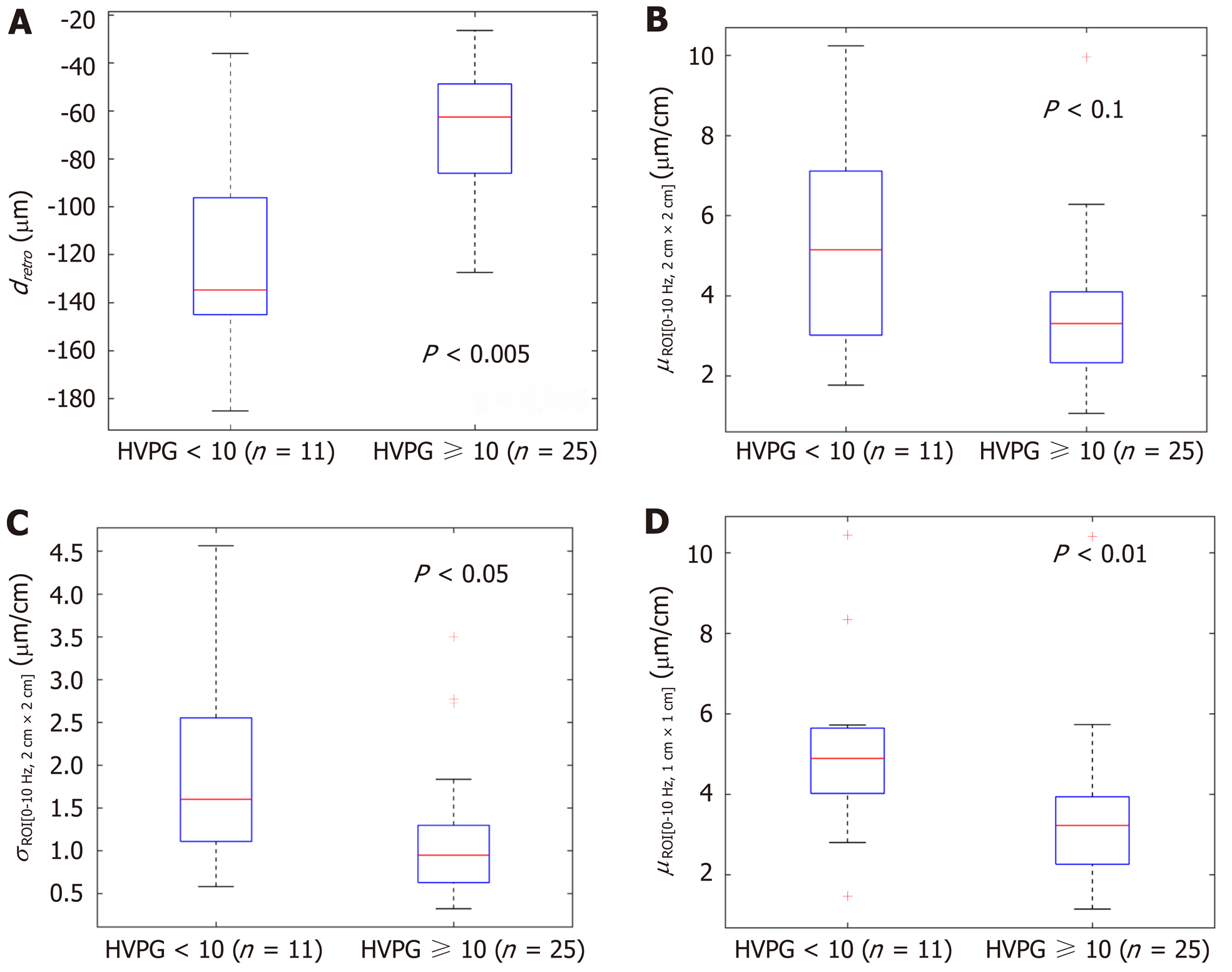
In the next stage we investigated the ability of these four parameters to evaluate CSPH (HVPG ≥ 10 mmHg) and SPH (HVPG ≥ 12 mmHg). The results are presented in boxplots as median and interquartile ranges (Figure 2 and 3). Three parameters (No. 2, 5, 6; Table 1) showed statistically significant differences between the groups.
According to ROC analysis dretro parameter had an AUC of 0.86 (P = 0.0001) for the diagnosis of CSPH and 0.84 (P = 0.0001) for the diagnosis of SPH. A cut-off value of -132.34 μm yielded 100% sensitivity for both conditions, whereas specificity was 80% and 72% for CSPH and SPH respectively (Figure 4). AUC of the σROI[0…10Hz, 2 cm × 2 cm] parameter reached 0.71 (P = 0.036) for the diagnosis of CSPH; with a cut-off value of 1.28 μm/cm providing 73% sensitivity and 70% specificity. AUC for the diagnosis of CSPH for µROI[0…10Hz, 1 cm × 1 cm] was 0.78 (P = 0.0024); with a cut-off value of 3.92 μm/cm providing 73% sensitivity and 80% specificity (Figure 5). Data on specificity, sensitivity, PPV and NPV are presented in Table 4.
| Parameter | Value | Sensitivity | Specificity | PPV | NPV | P value |
| dretro | ||||||
| CSPH | 132.34 µm | 100 | 80 | 93 | 100 | 0.0001 |
| SPH | 100 | 72 | 89 | 100 | 0.0001 | |
| σROI[0…10 Hz, 2 cm × 2 cm] | ||||||
| CSPH | 1.28 µm/cm | 73 | 70 | 86 | 50 | 0.036 |
| µROI[0…10 Hz, 1 cm × 1 cm] | ||||||
| CSPH | 3.92 µm/cm | 73 | 80 | 90 | 53 | 0.0024 |
Diagnosis of PH is currently based on the invasive procedure requiring high expertise; therefore, the need for an accurate non-invasive diagnostic test is growing. In this study for the non-invasive diagnosis of PH we applied a RF ultrasound-based tissue strain imaging method. We have shown that four out of 11 investigated parameters significantly correlated with HVPG and predicted CSPH and SPH. The dretro parameter was a strong predictor of CSPH and SPH with sensitivity and NPV of 100%. Other authors have described similar performance in non–invasive diagnosis of CSPH of TE[34], whereas pSWE and 2D SWE methods were less sensitive (AUC 0.85 and 0.81 respectively, sensitivity 71% and 85% respectively[35,36]).
The presented technique has certain limitations. It does not allow the evaluation of strain in real-time differently from commercial SE techniques and the strain parameters are calculated retrospectively, using recorded RF data. The other limitation of the approach is the detection of displacements field only in the direction of ultrasound wave propagation. Our method reveals the estimates of the displacements and strain only in single projection of endogenous motion, which is actually three-dimensional. The second projection of motion could be estimated by updating the algorithm for two-dimensional detection, but sector shaped scan requires complicated computations.
In this method we have used an undefined endogenous source for the tissue displacement excitation, which might influence quantified evaluation of liver elasticity. The endogenous motion also has a complex pattern which is composed from the very slow waves, induced by respiratory activity, a bit faster vascular pulsatility and relatively fast mechanical strain induced by the heartbeat and motion artefacts. However, the exact definition of motion inducing sources is beyond this study and will be investigated in future research. Other technical limitations of the method were disclosed in the previous study[32]. Also, despite the promising results, this was a pilot study and our results could be biased by the small size of our sample.
In conclusion, the parameters of endogenously induced displacements and strain of the liver significantly correlated with HVPG and could be a potential diagnostic tool for the non-invasive diagnosis of PH. A proposed method is potentially suitable for other applications of non-invasive diagnostics.
Degree of portal hypertension (PH) is the most important prognostic factor for the decompensation of liver cirrhosis and death, therefore adequate care for patients with liver cirrhosis requires timely detection and evaluation of the presence of clinically significant PH (CSPH) and severe PH (SPH).
As the most accurate method for the assessment of PH is an invasive direct measurement of hepatic venous pressure gradient (HVPG), the search for non-invasive methods to diagnose these conditions is actively ongoing. Ultrasound elastography is one of the most widely used non-invasive alternatives for the diagnosis of PH. Strain elastography (SE) is an ultrasound elastography technique widely used for the examination of musculoskeletal system, breast and thyroid pathologies, however it has been scarcely evaluated in the setting of liver cirrhosis and PH. It is an appealing alternative, as it is considered that SE readings are not affected by hepatic inflammation, jaundice, liver congestion, fatty degeneration, obesity, ascites or narrow intercostal spaces as is the case with other elastography modalities.
Our group has applied SE technique to assess the strain of liver tissue caused by endogenous motion of the beating heart and developed a specific radiofrequency (RF) signal analysis algorithm to calculate the parameters for quantification of strain in liver tissue. The aim of present study was to evaluate the ability and feasibility of endogenously induced displacements and strain on the liver to assess the degree of PH, using this specifically developed RF signal analysis algorithm.
Of 36 patients with liver cirrhosis and measured HVPG were included in the case-control study. Endogenous motion of the liver was characterized by derived parameters of region average tissue displacement signal (dantero, dretro, dRMS) and results of endogenous tissue strain imaging using specific radiofrequency signal processing algorithm. Average endogenous strain µ and standard deviation σ of strain were assessed in the regions of interest (ROI) (1 cm × 1 cm and 2 cm × 2 cm in size) and different frequency subbands of endogenous motion (0-10 Hz and 10-20 Hz).
Four parameters showed statistically significant (P < 0.05) correlation with HVPG measurement. The strongest correlation was obtained for the standard deviation of strain (estimated at 0-10 Hz and 2 cm × 2 cm ROI size). Three parameters showed statistically significant differences between patient groups with CSPH, but only dretro showed significant results in SPH analysis. According to ROC analysis area under the curve (AUC) of the σROI[0…10Hz, 2 cm × 2 cm] parameter reached 0.71 (P = 0.036) for the diagnosis of CSPH; with a cut-off value of 1.28 μm/cm providing 73% sensitivity and 70% specificity. AUC for the diagnosis of CSPH for µROI[0…10Hz, 1 cm × 1 cm] was 0.78 (P = 0.0024); with a cut-off value of 3.92 μm/cm providing 73% sensitivity and 80% specificity. Dretro parameter had an AUC of 0.86 (P = 0.0001) for the diagnosis of CSPH and 0.84 (P = 0.0001) for the diagnosis of SPH. A cut-off value of -132.34 μm yielded 100% sensitivity for both conditions, whereas specificity was 80% and 72% for CSPH and SPH respectively.
The parameters of endogenously induced displacements and strain of the liver significantly correlated with HVPG. This shows that parameters of endogenous motion could be a potential diagnostic tool for the non-invasive diagnosis of PH. A proposed method is potentially suitable for other applications of non-invasive diagnostics.
The presented technique has certain limitations. It does not allow the evaluation of strain in real-time differently from commercial SE techniques and the strain parameters are calculated retrospectively, using recorded RF data. The other limitation of the approach is the detection of displacements field only in the direction of ultrasound wave propagation. In this method we have used an undefined endogenous source for the tissue displacement excitation, which might influence quantified evaluation of liver elasticity. Also despite the promising results, this was a pilot study and our results could be biased by the small size of our sample. We are conducting further research of this method, which will include larger groups of patients, also allowing subgroup analysis of patients with ascites and obesity.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Corresponding Author's Membership in Professional Societies: Lithuanian Society of Gastroenterology.
P-Reviewer: Sun G S-Editor: Yan JP L-Editor: A P-Editor: Zhang YL
| 1. | Marcellin P, Kutala BK. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38 Suppl 1:2-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 287] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 2. | Snowdon VK, Guha N, Fallowfield JA. Noninvasive evaluation of portal hypertension: emerging tools and techniques. Int J Hepatol. 2012;2012:691089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Silkauskaitė V, Kupčinskas J, Pranculis A, Jonaitis L, Petrenkienė V, Kupčinskas L. Acute and 14-day hepatic venous pressure gradient response to carvedilol and nebivolol in patients with liver cirrhosis. Medicina (Kaunas). 2013;49:467-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Ravaioli F, Montagnani M, Lisotti A, Festi D, Mazzella G, Azzaroli F. Noninvasive Assessment of Portal Hypertension in Advanced Chronic Liver Disease: An Update. Gastroenterol Res Pract. 2018;2018:4202091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Marozas M, Zykus R, Sakalauskas A, Kupčinskas L, Lukoševičius A. Noninvasive Evaluation of Portal Hypertension Using a Supervised Learning Technique. J Healthc Eng. 2017;2017:6183714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Thabut D, Moreau R, Lebrec D. Noninvasive assessment of portal hypertension in patients with cirrhosis. Hepatology. 2011;53:683-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7:1303-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 954] [Cited by in F6Publishing: 871] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 8. | Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, Dietrich CF, Ding H, Amy D, Farrokh A, Ferraioli G, Filice C, Friedrich-Rust M, Nakashima K, Schafer F, Sporea I, Suzuki S, Wilson S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 557] [Article Influence: 61.9] [Reference Citation Analysis (1)] |
| 9. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Shi KQ, Fan YC, Pan ZZ, Lin XF, Liu WY, Chen YP, Zheng MH. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2013;33:62-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Şirli R, Sporea I, Popescu A, Dănilă M. Ultrasound-based elastography for the diagnosis of portal hypertension in cirrhotics. World J Gastroenterol. 2015;21:11542-11551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Zykus R, Jonaitis L, Petrenkienė V, Pranculis A, Kupčinskas L. Liver and spleen transient elastography predicts portal hypertension in patients with chronic liver disease: a prospective cohort study. BMC Gastroenterol. 2015;15:183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Kim SJ, Park HJ, Lee SY. Usefulness of strain elastography of the musculoskeletal system. Ultrasonography. 2016;35:104-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Fromageau J, Havre RF, Jenssen C, Ohlinger R, Săftoiu A, Schaefer F, Dietrich CF; EFSUMB. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 621] [Cited by in F6Publishing: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 15. | Kudo M, Shiina T, Moriyasu F, Iijima H, Tateishi R, Yada N, Fujimoto K, Morikawa H, Hirooka M, Sumino Y, Kumada T. JSUM ultrasound elastography practice guidelines: liver. J Med Ultrason (2001). 2013;40:325-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Carlsen JF, Ewertsen C, Lönn L, Nielsen MB. Strain Elastography Ultrasound: An Overview with Emphasis on Breast Cancer Diagnosis. Diagnostics (Basel). 2013;3:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Sandulescu L, Rogoveanu I, Gheonea IA, Cazacu S, Saftoiu A. Real-time elastography applications in liver pathology between expectations and results. J Gastrointestin Liver Dis. 2013;22:221-227. [PubMed] [Cited in This Article: ] |
| 18. | Dietrich CF, Barr RG, Farrokh A, Dighe M, Hocke M, Jenssen C, Dong Y, Saftoiu A, Havre RF. Strain Elastography - How To Do It? Ultrasound Int Open. 2017;3:E137-E149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Wu T, Ren J, Cong SZ, Meng FK, Yang H, Luo Y, Lin HJ, Sun Y, Wang XY, Pei SF, Zheng Y, He Y, Chen Y, Hu Y, Yang N, Li P, Kudo M, Zheng RQ. Accuracy of real-time tissue elastography for the evaluation of hepatic fibrosis in patients with chronic hepatitis B: a prospective multicenter study. Dig Dis. 2014;32:791-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Morikawa H, Fukuda K, Kobayashi S, Fujii H, Iwai S, Enomoto M, Tamori A, Sakaguchi H, Kawada N. Real-time tissue elastography as a tool for the noninvasive assessment of liver stiffness in patients with chronic hepatitis C. J Gastroenterol. 2011;46:350-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Mobarak L, Nabeel MM, Hassan E, Omran D, Zakaria Z. Real-time elastography as a noninvasive assessment of liver fibrosis in chronic hepatitis C Egyptian patients: a prospective study. Ann Gastroenterol. 2016;29:358-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 257] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 23. | Ge L, Shi B, Song YE, Li Y, Wang S, Wang X. Clinical value of real-time elastography quantitative parameters in evaluating the stage of liver fibrosis and cirrhosis. Exp Ther Med. 2015;10:983-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Thiele M, Kjaergaard M, Thielsen P, Krag A. Contemporary use of elastography in liver fibrosis and portal hypertension. Clin Physiol Funct Imaging. 2017;37:235-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Piscaglia F, Marinelli S, Bota S, Serra C, Venerandi L, Leoni S, Salvatore V. The role of ultrasound elastographic techniques in chronic liver disease: current status and future perspectives. Eur J Radiol. 2014;83:450-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Koizumi Y, Hirooka M, Abe M, Tokumoto Y, Yoshida O, Watanabe T, Nakamura Y, Imai Y, Yukimoto A, Kumagi T, Takeshita E, Ikeda Y, Hiasa Y. Comparison between real-time tissue elastography and vibration-controlled transient elastography for the assessment of liver fibrosis and disease progression in patients with primary biliary cholangitis. Hepatol Res. 2017;47:1252-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Sporea I, Gilja OH, Bota S, Şirli R, Popescu A. Liver elastography - an update. Med Ultrason. 2013;15:304-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Wu T, Wang P, Zhang T, Zheng J, Li S, Zeng J, Kudo M, Zheng R. Comparison of Two-Dimensional Shear Wave Elastography and Real-Time Tissue Elastography for Assessing Liver Fibrosis in Chronic Hepatitis B. Dig Dis. 2016;34:640-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Ochi H, Hirooka M, Koizumi Y, Miyake T, Tokumoto Y, Soga Y, Tada F, Abe M, Hiasa Y, Onji M. Real-time tissue elastography for evaluation of hepatic fibrosis and portal hypertension in nonalcoholic fatty liver diseases. Hepatology. 2012;56:1271-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Hirooka M, Ochi H, Koizumi Y, Kisaka Y, Abe M, Ikeda Y, Matsuura B, Hiasa Y, Onji M. Splenic elasticity measured with real-time tissue elastography is a marker of portal hypertension. Radiology. 2011;261:960-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Sakalauskas A, Jurkonis R, Gelman S, Lukoševičius A, Kupčinskas L. Development of radiofrequency ultrasound based method for elasticity characterization using low frequency endogenous motion: Phantom study. In: Eskola H, Väisänen O, Viik J, Hyttinen J, editors. EMBEC & NBC 2017: IFMBE Proceedings; 2017 June 13. Singapore: Springer, 2017; 65. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Sakalauskas A, Jurkonis R, Gelman S, Lukoševičius A, Kupčinskas L. Investigation of Radiofrequency Ultrasound-Based Fibrotic Tissue Strain Imaging Method Employing Endogenous Motion. J Ultrasound Med. 2019;38:2315-2327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 34. | Song J, Ma Z, Huang J, Luo Y, Zykus R, Kumar A, Kitson M, Lu Q. Reliability of Transient Elastography-Based Liver Stiffness for Diagnosing Portal Hypertension in Patients with Alcoholic Liver Disease: A Diagnostic Meta-Analysis with Specific Cut-Off Values. Ultraschall Med. 2020;41:60-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Salzl P, Reiberger T, Ferlitsch M, Payer BA, Schwengerer B, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Evaluation of portal hypertension and varices by acoustic radiation force impulse imaging of the liver compared to transient elastography and AST to platelet ratio index. Ultraschall Med. 2014;35:528-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Kim TY, Jeong WK, Sohn JH, Kim J, Kim MY, Kim Y. Evaluation of portal hypertension by real-time shear wave elastography in cirrhotic patients. Liver Int. 2015;35:2416-2424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |