Copyright
©The Author(s) 2020.
World J Gastroenterol. Aug 21, 2020; 26(31): 4589-4606
Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4589
Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4589
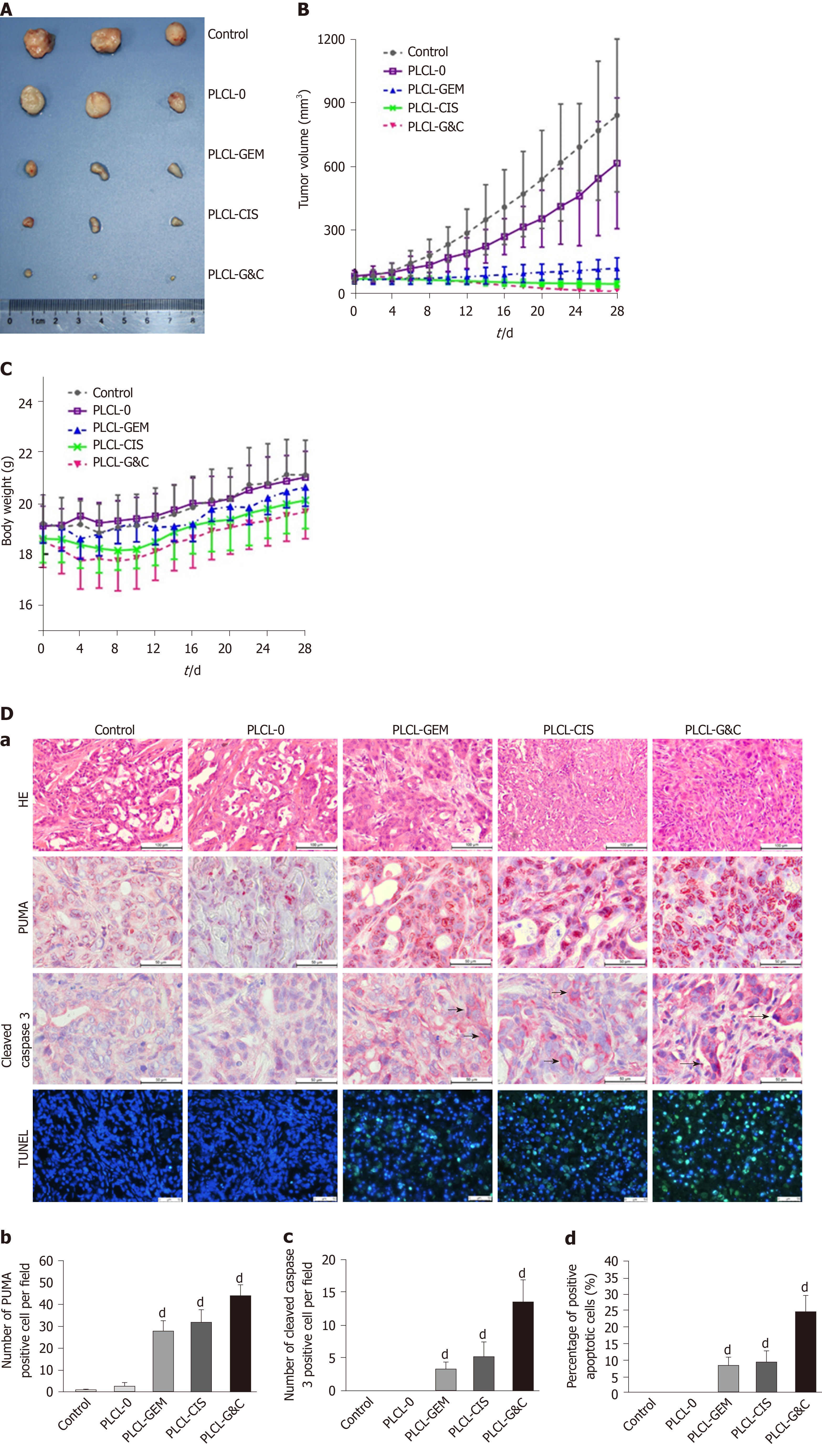
Figure 4 Antitumor activity of drug-loaded nanofilms against subcutaneous tumor xenografts in nude mice in vivo.
A: Image of subcutaneous tumors from nude mice after drug-loaded nanofilm implantation for 4 wk; B: Changes in tumor volume; C: Changes in body weight; and D: (a) HE staining (magnification × 200, scale bar 100 µm), immunohistochemical analysis of p53 upregulated modulator of apoptosis and cleaved caspase-3 (×400, scale bar 50 µm), and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining (× 200, scale bar 50 µm) in subcutaneous tumor tissues; (b-d) Statistical analysis results: Quantification of (b) p53 upregulated modulator of apoptosis-positive cell numbers, (c) cleaved caspase-3-positive cell numbers, and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling-positive cell proportion in subcutaneous tumor tissues. dP < 0.001 vs control (n = 6 mice per group). CIS: Cisplatin; GEM: Gemcitabine; HE: Hematoxylin and eosin; PLCL: Poly L-lactide-caprolactone; PLCL-0: Non-drug-loaded PLCL nanofilm; PLCL-CIS: PLCL nanofilm loaded with CIS; PLCL-GEM: PLCL nanofilm loaded with GEM; PLCL-GC: PLCL nanofilm loaded with both GEM and CIS; PUMA: p53 upregulated modulator of apoptosis; TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
- Citation: Xiao JB, Weng JY, Hu YY, Deng GL, Wan XJ. Feasibility and efficacy evaluation of metallic biliary stents eluting gemcitabine and cisplatin for extrahepatic cholangiocarcinoma. World J Gastroenterol 2020; 26(31): 4589-4606
- URL: https://www.wjgnet.com/1007-9327/full/v26/i31/4589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i31.4589