Copyright
©The Author(s) 2020.
World J Gastroenterol. Aug 21, 2020; 26(31): 4589-4606
Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4589
Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4589
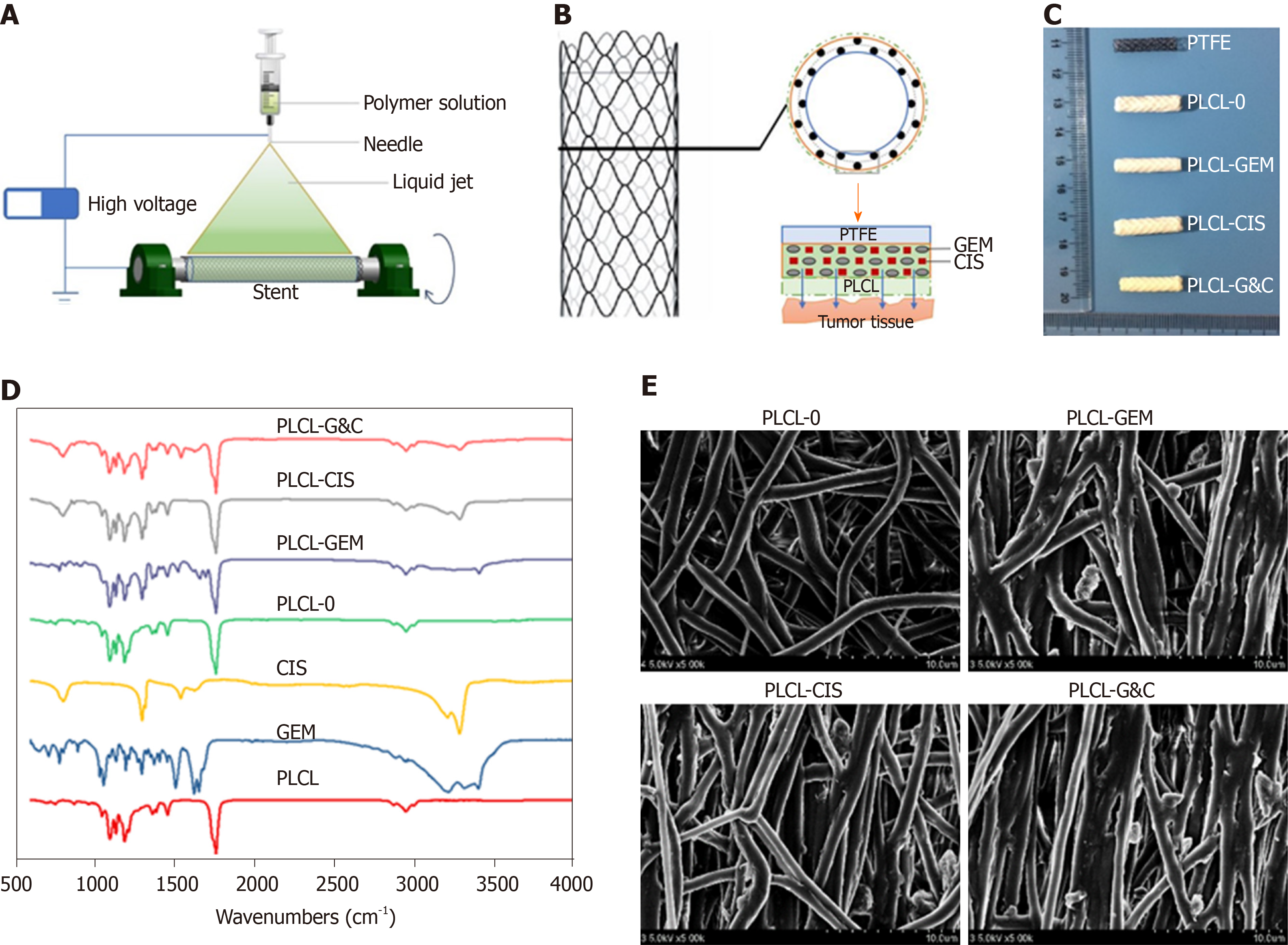
Figure 1 Manufacturing and characteristics of different biliary drug-eluting stents.
A: Schematic illustration of the manufacturing of drug-eluting stents by the mixed electrospinning method; B: Optimized design of the gemcitabine/cisplatin-eluting biliary stent. The entire structure of the coated membranes consists of three layers: the inner polytetrafluoroethylene membrane (as a blocker to avoid drug loss), the middle PLCL layer (as the major drug carrier), and the outer PLCL-unloaded membrane (as a blocker to prevent burst release); C: Image of different types of drug-eluting stents; D: Fourier transform infrared spectra of different prototype drugs and drug-eluting nanofilms; E: Scanning electron microscope images of different drug-eluting nanofilms (magnification ×2000). CIS: Cisplatin; GEM: Gemcitabine; PLCL: Poly-L-lactide-caprolactone; PLCL-0: Non-drug-loaded PLCL nanofilm; PLCL-CIS: PLCL nanofilm loaded with CIS; PLCL-GEM: PLCL nanofilm loaded with GEM; PLCL-GC: PLCL nanofilm loaded with both GEM and CIS; PTFE: Polytetrafluoroethylene.
- Citation: Xiao JB, Weng JY, Hu YY, Deng GL, Wan XJ. Feasibility and efficacy evaluation of metallic biliary stents eluting gemcitabine and cisplatin for extrahepatic cholangiocarcinoma. World J Gastroenterol 2020; 26(31): 4589-4606
- URL: https://www.wjgnet.com/1007-9327/full/v26/i31/4589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i31.4589