Published online Jun 7, 2020. doi: 10.3748/wjg.v26.i21.2810
Peer-review started: February 17, 2020
First decision: March 30, 2020
Revised: March 30, 2020
Accepted: April 27, 2020
Article in press: April 27, 2020
Published online: June 7, 2020
Liver fibrosis is a common health problem worldwide and there is still a lack of effective medicines. The Chinese herbal medicine, Gan Shen Fu Fang (GSFF) is composed of salvianolic acid B and diammonium glycyrrhizinate. In this study, we observed the effects of GSFF on liver fibrosis in vivo and in vitro in an attempt to provide some hope for the treatment.
To observe the effects of GSFF on liver fibrosis in vivo and in vitro and investigate the mechanism from the perspective of the inflammatory response and extracellular signal-regulated kinase (ERK) phosphorylation.
Common bile duct-ligated rats were used for in vivo experiments. Hepatic stellate cells-T6 (HSC-T6) cells were used for in vitro experiments. Hematoxylin and eosin staining and Masson staining, biochemical assays, hydroxyproline (Hyp) assays, enzyme-linked immunoasorbent assay and western blotting were performed to evaluate the degree of liver fibrosis, liver function, the inflammatory response and ERK phosphorylation. The CCK8 assay, immunofluorescence and western blotting were applied to test the effect of GSFF on HSC-T6 cell activation and determine whether GSFF had an effect on ERK phosphorylation in HSC-T6 cells.
GSFF improved liver function and inhibited liver fibrosis in common bile duct-ligated rats after 3 wk of treatment, as demonstrated by histological changes, hydroxyproline assays and collagen I concentrations. GSFF alleviated inflammatory cell infiltration and reduced the synthesis of pro-inflammatory cytokines [tumor necrosis factor-α (TNF-α) and interlukin-1β] and NF-κB. In addition, GSFF decreased ERK phosphorylation. In vitro, GSFF inhibited the viability of HSC-T6 cells with and without transforming growth factor β1 (TGF-β1) stimulation and decreased the synthesis of collagen I. GSFF had the greatest effect at a concentration of 0.5 μmol/L. GSFF inhibited the expression of α-smooth muscle actin (α-SMA), a marker of HSC activation, in HSC-T6 cells. Consistent with the in vivo results, GSFF also inhibited the phosphorylation of ERK and downregulated the expression of NF-κB.
GSFF inhibited liver fibrosis progression in vivo and HSC-T6 cell activation in vitro. These effects may be related to an alleviated inflammatory response and downregulated ERK phosphorylation.
Core tip: Liver fibrosis results from various kinds of chronic liver diseases and there is no specific treatment so far. Inflammatory response and extracellular signal-regulated kinase cascade play an important role in liver fibrosis development and progression. In this study, we observed the effects of herbal medicine, Gan Shen Fu Fang (GSFF) on liver fibrosis in vivo and in vitro. The results indicate that GSFF alleviates liver fibrosis progression in vivo and inhibits HSC-T6 activation in vitro, which may be related with inhibited inflammatory response and downregulated extracellular signal-regulated kinase phosphorylation. GSFF may provide hope for liver fibrosis treatment.
- Citation: Du QH, Zhang CJ, Li WH, Mu Y, Xu Y, Lowe S, Han L, Yu X, Wang SY, Li Y, Li J. Gan Shen Fu Fang ameliorates liver fibrosis in vitro and in vivo by inhibiting the inflammatory response and extracellular signal-regulated kinase phosphorylation. World J Gastroenterol 2020; 26(21): 2810-2820
- URL: https://www.wjgnet.com/1007-9327/full/v26/i21/2810.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i21.2810
Liver fibrosis, characterized by the excessive deposition of extracellular matrix (ECM), is the common result of various kinds of chronic liver diseases (chronic viral hepatitis, alcoholic hepatitis, nonalcoholic fatty liver disease, etc.). Liver fibrosis progresses and ultimately leads to liver cirrhosis. Cirrhosis is characterized by the replacement of normal liver with fibrous septa, which disrupts the normal liver architecture and causes the formation of numerous abnormal nodules. Liver cirrhosis can induce severe consequences, such as ascites, splenomegaly, collateral circulation formation, and even upper gastrointestinal bleeding that can lead to death[1]. Therefore, exploring the mechanism of liver fibrosis and developing effective and safe anti-fibrosis medicines remains a research focus.
Hepatic stellate cells (HSCs), which are located in the disse space, are a kind of nonparenchymal cell. HSCs maintain a quiescent phenotype as fat-storing cells in the body[2]. Once the liver is injured, HSCs undergo dramatic phenotypic transformation. HSCs become activated and trans-differentiate into myofibroblasts, which are characterized by increased cell proliferation, survival, α-SMA expression, and ECM production (including collagen I, collagen III and fibronectin)[3]. Thus, activation of HSCs is a central event in liver fibrosis[4,5]. Inflammation is present in virtually all patients with liver fibrosis and correlated with fibrosis progression. The chronic inflammatory response is believed to sustain chronic liver disease progression[6,7]. Inflammation has been demonstrated to activate quiescent HSCs into myofibroblasts through enhanced TGF-β signalling[8]. In addition, activated macrophages, which participate in the inflammatory response induced by aseptic or septic stimuli, release TGF-β to promote ECM synthesis by activated HSCs[9]. Therefore, one strategy to alleviate liver fibrosis is regulation of the inflammatory response. NF-κB activation in macrophages and HSCs is a key precipitating factor that increases pro-inflammatory mediators, such as TNF-α and interlukin-1β. Such cytokines regulate inflammation, immune responses and cell survival in hepatic fibrosis[10,11].
The extracellular signal-regulated kinase (ERK) cascade, which is a critical MAPK signalling pathway, plays a major role in liver fibrogenesis. The extracellular signals that stimulate the ERK cascade include platelet-derived growth factor (PDGF), TGF-β, epidermal growth factor (EGF), and reactive oxygen species (ROS)[12]. Once activated, the signal is transmitted through the sequential phosphorylation and activation of sequential kinases. The phosphorylation of hundreds of substrates results in the induction of several extracellular signal-regulated kinase1/2(ERK1/2)-dependent processes. In liver fibrosis, the ERK cascade is strongly related to HSC activation and has been shown to lead to the increased proliferation and survival of HSCs[13,14], synthesis of ECM[15], and plays pro-inflammatory, immune-modulatory[7], and pro-angiogenic roles[16]. Therefore, drugs and strategies designed to target the ERK1/2 signalling pathway provide hope for anti-fibrotic treatment.
Gan Shen Fu Fang (GSFF) (previously named Glytan), a Chinese herbal medicine, is composed of salvianolic acid B (SA-B) and diammonium glycyrrhizinate (DG). SA-B is extracted from Salvia miltiorrhiza and DG is from liquorice. Salvia miltiorrhiza and liquorice are commonly used herbal medicines in China. The molecular formulas of both SA-B and DG have been established. Previous studies on GSFF focused on its role in portal hypertension[17]. We have shown that GSFF could reduce portal pressure and portal territory blood flow and increase mean arterial pressure and splanchnic vascular resistance in common bile duct-ligated (CBDL) rats[18]. Because of GSFF’s significant effect on decreasing portal pressure, clinical trial was approved by the FDA of China in 2015. During pre-clinical experiment, we found that GSFF could inhibit pseudo-lobule formation, restore the fenestrae of liver sinusoidal endothelial cells and reverse hepatic sinusoid capillarization in CBDL rats. Therefore, in this study, we aimed to observe the effects of GSFF on liver fibrosis in vivo and in vitro and explore whether GSFF-mediated alleviation of liver fibrosis is related to inflammation and the ERK signalling pathway.
For the animal experiment, male Sprague-Dawley (SD) rats (SPF, Biotechnology Co., Ltd., Beijing, China) weighing 250-280 g were randomly divided into 3 groups: Sham group, CBDL group, and GSFF group. The rats underwent sham surgery or common bile duct ligation. The common bile ducts were exposed and ligated twice. The segment between the two ligations was resected, and the abdomen was sutured. The common bile ducts of sham rats were exposed but did not undergo ligation or resection. All experimental procedures were conducted in accordance with the guidelines for the use of experimental animals and were approved by the Institutional Review Committee on Animal Care and Use at the Experimental Animal Centre of Beijing University of Chinese Medicine [certificate of conformity: SCXK (2012-0001)].
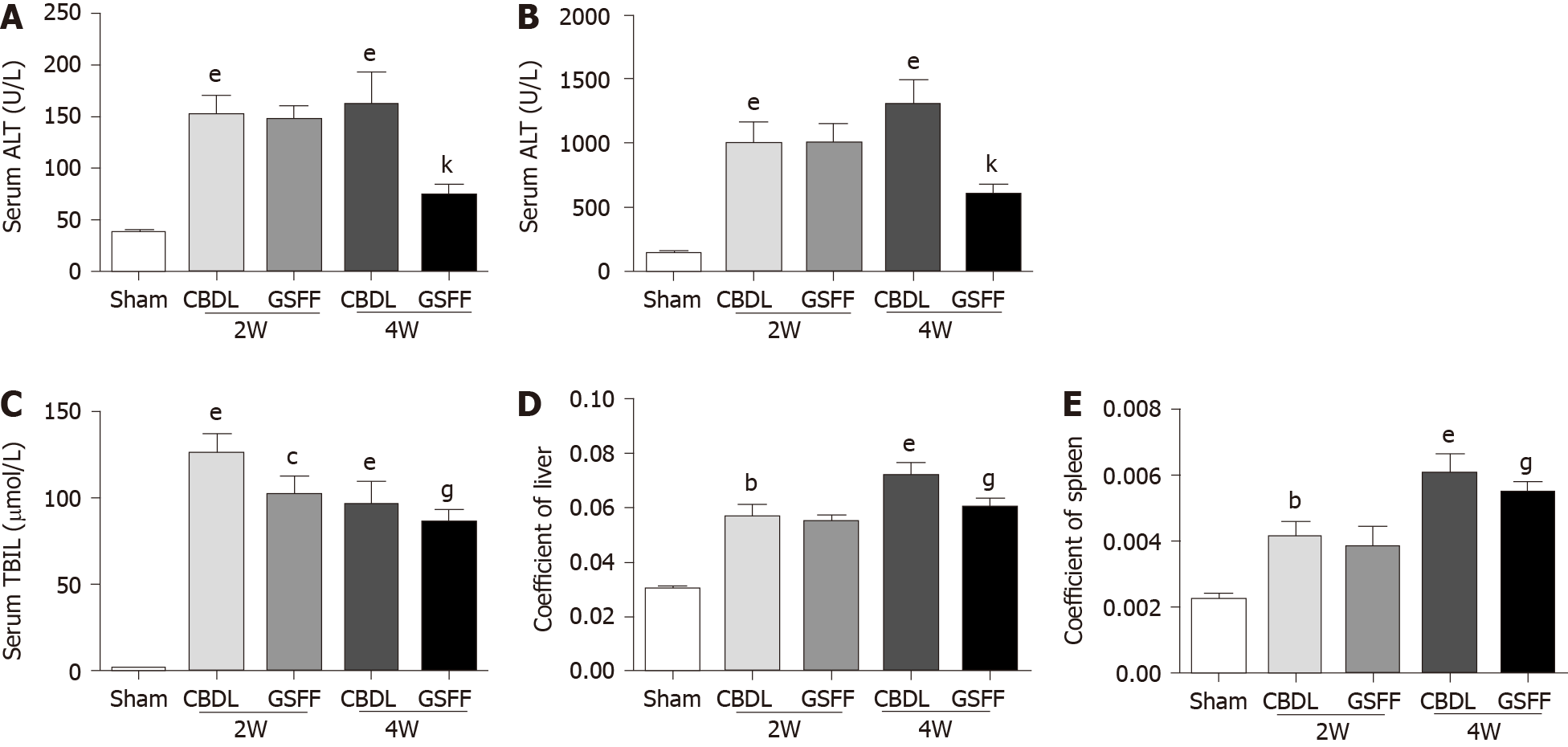
SA-B (115939-25-8, purity ≥ 98%, Aladdin Biochemical Technology Co., Ltd.) and DG (s101148, purity ≥ 98%, Nature Standard Technical Service Co., Ltd.) were used at a ratio of 1:1. Before use, SA-B and DG were diluted with distilled water. After 1 wk of CBDL, rats in the GSFF group were treated with GSFF (25 mg/kg/d) by gavage. The dosage of GSFF depended on previous pharmacokinetic experimental results[18]. Rats in the sham and CBDL groups were administered the same amount of distilled water. The rats were sacrificed at 2 and 4 wk. Liver, spleen and body weights were recorded and used to calculate the liver and spleen coefficients as follows: Liver or spleen coefficient = liver or spleen weight/body weight. Liver tissues and blood were collected for subsequent analysis.
The HSC-T6 rat HSC line (purchased from Kunming Cell Bank, Chinese Academy of Sciences) was cultured in high-glucose DMEM containing 10% FBS at 37°C with 5%CO2. First, to observe the effect of GSFF on HSC-T6 cell viability, based on some previous studies[19,20], we chose and used a gradient of 6 concentrations, 0.03125, 0.0625, 0.125, 0.25, 0.5, and 1 μmol/L, to select the appropriate concentration of GSFF to inhibit HSC-T6 cell viability. Second, to further verify the effect of GSFF on HSC-T6 cell viability, HSC-T6 cells were stimulated with TGF-β1 (2 ng/mL, Peprotech, United States) for 1 h, and the cells were then treated with GSFF. Third, to evaluate the effect of GSFF on ERK, HSC-T6 cells were pre-treated with PDGF-BB (10 ng/mL, Peprotech) for 1 h and then incubated with the ERK antagonist PD98059 (50 μmol/L, Abmole) or 0.125, 0.25, or 0.5 μmol/L GSFF for 24 h. Cells were starved of serum for 12 h before stimulation.
Hematoxylin and eosin staining and Masson staining were performed according to our previous protocol[18]. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) levels in the serum were measured using a commercial colorimetric kit (Randox Laboratories, Antrim, United Kingdom). The hydroxyproline (Hyp) content in the liver was measured with Hyp assay kits (Nanjing JianCheng, China). The Hyp content is expressed as micrograms of Hyp per milligram of wet liver weight.
The concentration of collagen I (Col. I) in the culture supernatant of HSC-T6 cells was analysed using a commercial enzyme-linked immunoasorbent assay kit (CSB-E09243r, S-ABC kit). The concentrations of TNF-α and IL-1β in the liver tissue were analyzed according to the manufacturer's protocol.
The CCK-8 assay was performed to test cell viability. HSC-T6 cells were seeded in 96-well plates at 5 × 104 cells/mL. After 24 h, the cells were treated with different concentrations of GSFF for 24 h. Subsequently, 10 μL of CCK-8 reagent was added to each well, followed by incubation at 37°C for 2 h. To further observe the inhibitory effect of GSFF on HSC-T6 cell activation, HSC-T6 cells were stimulated with TGF-β1 (2 ng/mL) for 1 h as described before and then treated with 0.125, 0.25, or 0.5 μmol/L GSFF for 24h. The absorbance at 450 nm was measured with a microplate reader (Bio-Rad, United States).
Cells were seeded at a density of 2 × 105 cells/mL in laser confocal dishes. After 24 h, the HSC-T6 cells were stimulated with TGF-β1 for 1 h, and the cells were then treated with 0.125, 0.25, or 0.5 μmol/L GSFF for 24 h. The following day, the cells were fixed with 4% paraformaldehyde for 20 min. Then, the cells were treated with anti α-SMA antibody (1:50, a gift from Christine Chaponnier, Geneva University) overnight at 4°C and incubated for 1 h with a secondary antibody (Alexa Fluor-594 donkey-anti-rabbit IgG secondary antibody). Finally, nuclei were stained with DAPI (1:1000) for 2 min in the dark. Images were taken using a confocal microscope (Olympus Fv1000). The percentage of α-SMA-positive cells was determined using Image J software.
Col.I, NF-κB, ERK, and p-ERK protein expression levels were detected by western blotting as previously described[18]. In brief, liver samples or cell lysates were scraped in ice-cold lysis buffer. The protein content was quantified with a BCA protein assay reagent kit. Total liver and cell lysates were separated on 10% polyacrylamide gels by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. After blocking in a 5% nonfat powdered milk solution or bovine serum albumin for 1 h, the membranes were incubated with primary antibodies overnight at 4°C. The following primary antibodies were used for western blotting: mouse anti-collagen I (ab90395, Abcam, United States, 1:4000), rabbit anti-rat monoclonal NF-κB (p65) (8242S, Cell Signaling, United States, 1:4000), rabbit anti-rat monoclonal ERK (16443-1-AP, Proteintech Group, United States, 1:4000), and rabbit anti-rat monoclonal phospho-p44/42 mitogen-activated protein kinase (Erk1/2) (4370S, Cell Signaling Technology, MA, United States, 1:6000). Then, the membranes were incubated with the appropriate secondary antibody for 2 h at room temperature. GAPDH (60004-1-Ig, Proteintech Group, United States, 1:40000) was used as an internal reference/control.
The experimental data were analysed using SPSS 22.0 statistical software, and the data are expressed as the mean ± SD. To compare differences in groups of data with a normal distribution and uniform variance, one-way ANOVA was used; if the data did not conform to a normal distribution or the variance was not uniform, a nonparametric test was used. P < 0.05 indicated a statistically significant difference.
After 2 and 4 wk of CBDL, ALT, AST and TBIL were increased significantly in CBDL rats compared with sham rats, which indicated hepatocyte injury after cholestasis (Figure 1A-C). GSFF decreased ALT, AST and TBIL levels at 4 wk, but not at 2 wk (Figure 1A-C). In addition, GSFF reduced the liver and spleen coefficients at 2 and 4 wk (Figure 1D and E).
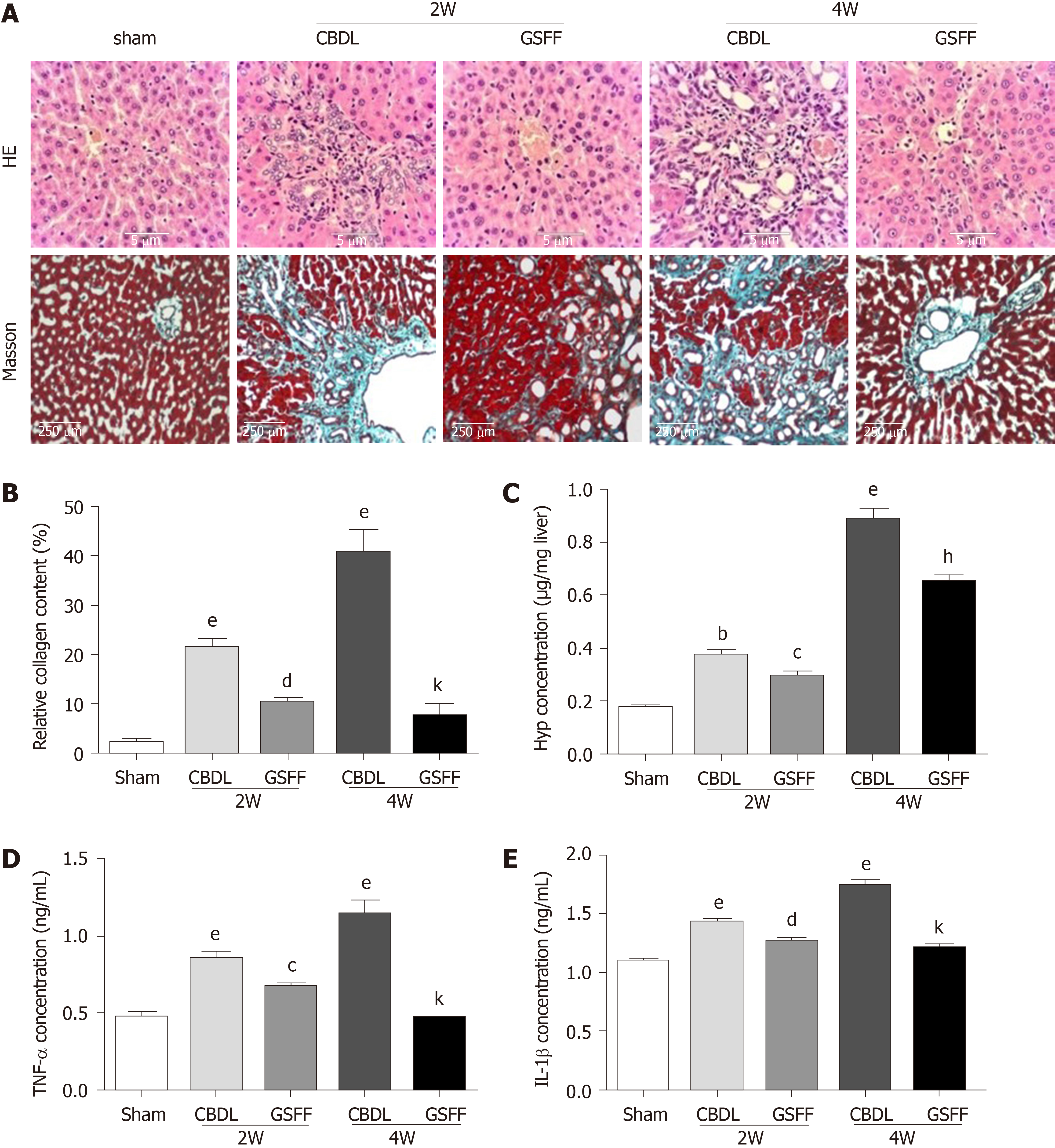
Hematoxylin and eosin staining showed a normal liver structure and hepatic cords arranged radially around the central vein in rats in the sham group (Figure 2A). After 2 wk, the proliferation of small bile ducts in CBDL rats was clearly observed. In addition, many fibroblasts and inflammatory cells had infiltrated to the area around the newly proliferated bile ducts. After 4 wk, with the progression of cholestasis, bile duct proliferation in CBDL rats was more severe, and a destroyed histological structure, the infiltration of a large number of inflammatory cells and fibrotic septa formation were observed. The increased inflammatory response was also confirmed by increased production of the cytokines TNF-α and IL-1β (Figure 2D and E). Masson staining indicated the extensive proliferation of fibrotic tissue in CBDL rats after 2 and 4 wk (Figure 2A and B). Particularly at 4 wk, the fibrotic tissue was linked, and the normal liver structure had been destroyed. Compared with CBDL rats, GSFF-treated rats showed alleviated bile duct proliferation, reduced inflammatory cell infiltration and TNF-α and IL-1β synthesis, and decreased fibrotic tissue accumulation after 2 and 4 wk of CBDL. Consistent with the results of Masson staining, the Hyp concentration was increased after 2 and 4 wk of CBDL, and GSFF reduced Hyp synthesis (Figure 2C).
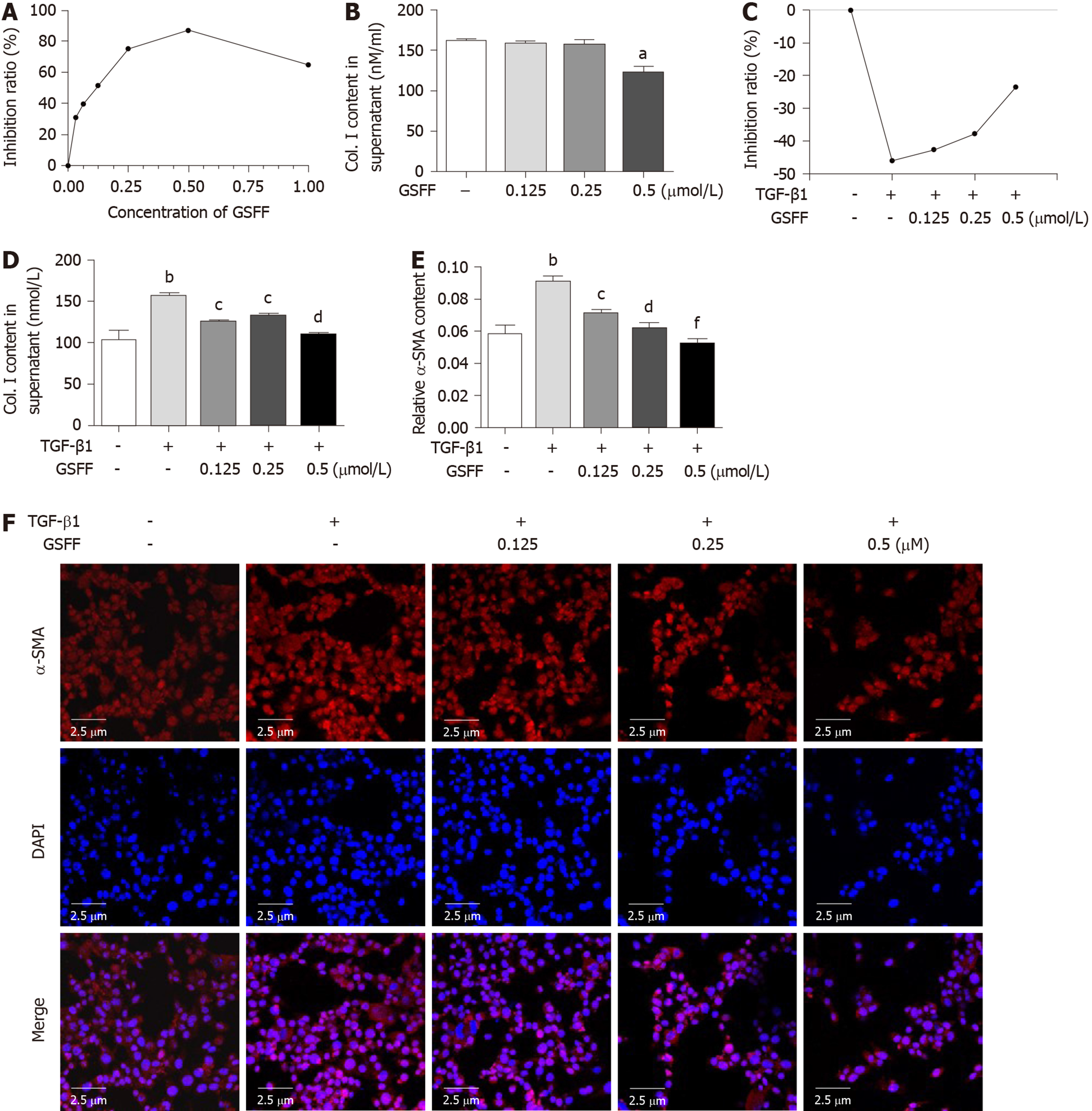
Treatment with 0.03125, 0.0625, 0.125, 0.25, 0.5, and 1 μmol/L GSFF for 24 h had an obvious inhibitory effect on the viability of HSC-T6 cells (Figure 3A). From this result, we identified the following two points. First, GSFF at a concentration from 0.03125 to 0.5 μmol/L had a substantial dose-dependent inhibitory effect. In particular, the dose-dependent effect of GSFF at 0.125, 0.25, and 0.5μmol/L was evident (P < 0.001). Second, although 1 μmol/L GSFF also inhibited cell viability, the effect of GSFF at this dose was not as pronounced as that of GSFF at 0.5 μmol/L. We also tested the effect of GSFF at other doses (5 μmol/L, 10 μmol/L) and found that the inhibitory effect of GSFF was decreased with increasing dose (data not shown). Based on these results, GSFF at concentrations of 0.125, 0.25, and 0.5 μmol/L was selected for the following experiments. The enzyme-linked immunoasorbent assay results showed that 0.125, 0.25, and 0.5 μmol/L GSFF could inhibit their release of Col.I and that the effect of 0.5 μmol/LGSFF was most obvious (Figure 3B).
To further confirm the effect of GSFF on HSC-T6 cell activation, the groups of cells except the control cells were stimulated with TGF-β1. Cell viability and proliferation were significantly promoted by TGF-β1. Treatment with GSFF at 0.125, 0.25 and 0.5 μmol/L inhibited cell viability more than treatment with TGF-β1 alone (Figure 3C). Both Col. I synthesis and α-SMA expression were significantly increased after stimulation with TGF-β1 (Figure 3D-F), and GSFF could downregulate collagen synthesis and α-SMA expression, with the effect being most obvious at a concentration of 0.5 μmol/L.
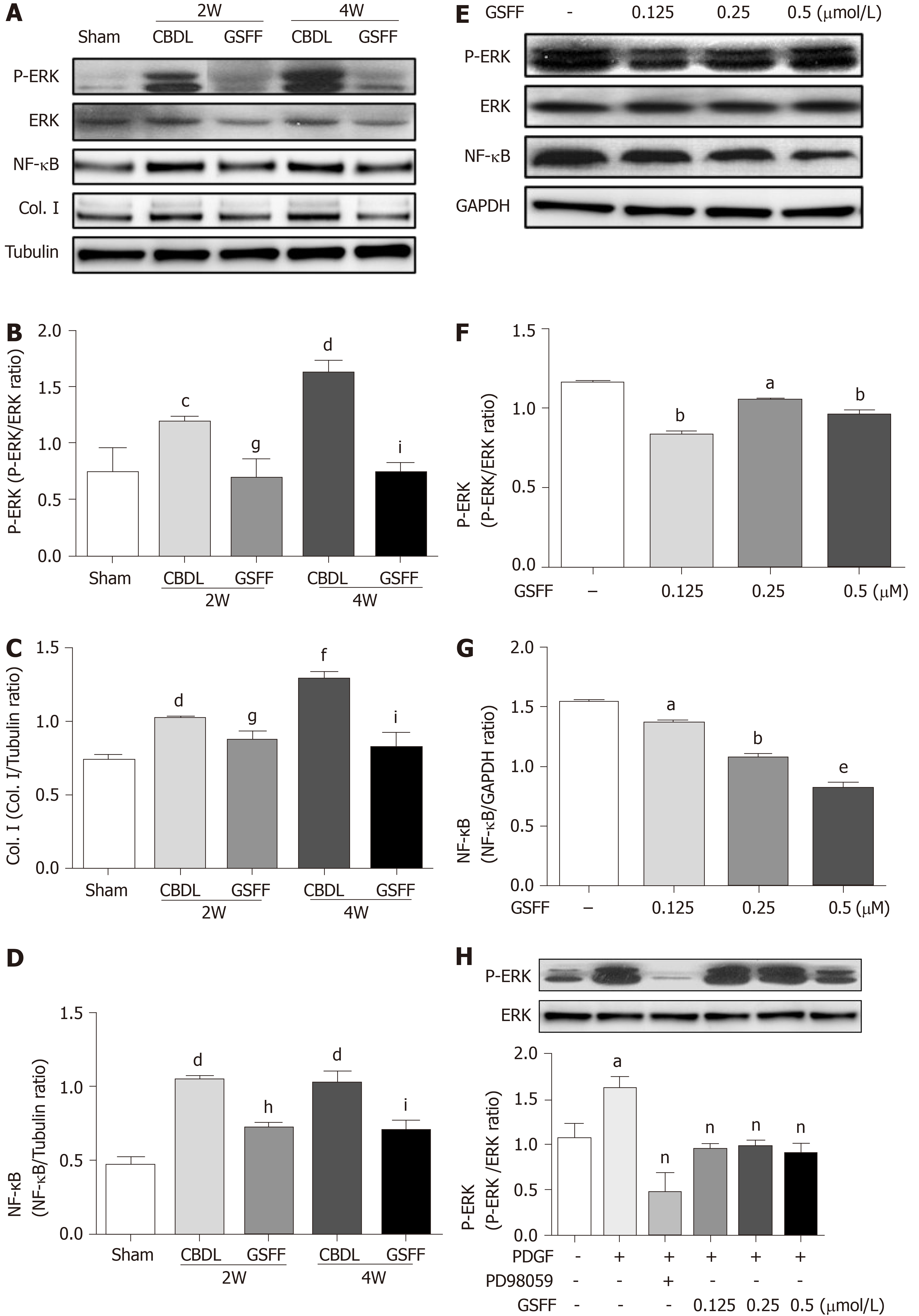
NF-κB is an important mediator involved in the inflammatory responses of various organs. In this study, after 2 and 4 wk of CBDL, the level of NF-κB (p65) expression was increased in the CBDL rats, and GSFF inhibited NF-κB (p65) expression (Figure 4A and D).The levels of p-ERK were dramatically increased in the livers of CBDL rats. GSFF inhibited p-ERK expression at 2 and 4 wk (Figure 4A and B). Col. I synthesis was also examined by western blotting and found to be significantly increased after CBDL and inhibited by GSFF at 2 and 4 wk (Figure 4A and C).
In vitro, GSFF inhibited ERK phosphorylation in HSC-T6 cells (Figure 4E and F). After stimulating the cells with PDGF-BB, ERK phosphorylation was increased and inhibited by PD98059 and GSFF, respectively (Figure 4H). GSFF also inhibited the expression of NF-κB (p65) in HSC-T6 cells (Figure 4E and G).
Liver fibrosis, the result of most types of chronic liver diseases, is a common health problem worldwide. Though important progress has been made in basic research on liver fibrosis, there is still a lack of effective clinical medicines. In this study, we showed that the herbal medicine GSFF alleviated liver fibrosis and inhibited HSC-T6 cell activation, which were related to a decreased inflammatory response and reduced ERK phosphorylation.
First, GSFF was shown to inhibit liver fibrosis progression and attenuate cholestatic liver injury. Common bile duct ligation in rats or mice is a classic method to produce an animal model of liver fibrosis[21-23]. When bile was blocked within the livers of our model rats, hepatocytes underwent necrosis. To repair this injury, tissue damage and concomitant inflammation triggered fibrotic tissue proliferation and lead to excessive ECM accumulation, as evidenced by histological changes; increased serum ALT, AST, and TBIL levels; and increased Hyp content in the liver tissue after 2 and 4 wk of CBDL.
GSFF comprises two ingredients, SA-B and DG. SA-B was shown to inhibit liver fibrosis induced by CCl4[24]. DG was found to have prominent anti-inflammatory effects and improve liver function. Diammonium glycyrrhizin (DG, known as Gan Li Xin in China) is commonly used to treat chronic viral hepatitis. Hyp assays and Masson staining verified the anti-fibrotic effect of GSFF after 2 and 4 wk of treatment. GSFF decreased ALT, AST and TBIL levels at 4 wk but not at 2 wk. This may be because the 1-wk GSFF treatment time was too short. A longer 3-wk GSFF treatment time was long enough for GSFF to protect hepatocytes. In this study, we also found that the livers and spleens of CBDL rats were larger than those of the sham rats and had shrunk after GSFF treatment. Enlargement of the liver and spleen may have resulted from inflammatory congestion and cholestasis.
Second, GSFF inhibited HSC activation, as indicated by their reduced viability and collagen synthesis. Activated HSCs experience structural and functional changes and are the main source of myofibroblasts in liver fibrosis[25,26]. Functionally, activated HSCs acquire enhanced viability (hyper-proliferation, hyper-migration) and exhibit the increased secretion of ECM, which is very important in liver fibrosis formation and progression[27,28]. Because the effect of GSFF on HSCs had not been tested in vitro, we first designed a 6-concentration gradient of GSFF and used this concentration series to determine the dose of GSFF with an effect on HSC activation. GSFF at concentrations of 0.125, 0.25 0.5 μmol/L was selected for subsequent experiments. We found that 0.125, 0.25, and 0.5 μmol/L GSFF inhibited HSC-T6 cell viability and Col.I synthesis with and without stimulation by TGF-β1. In addition, the effect of GSFF was most pronounced at a concentration of 0.5 μmol/L. Zhang et al[29] found that SA-B (1 μmol/L) inhibited the expression of α-SMA and Col. I in human HSCs. However, in this study, though 1μmol/L GSFF (containing 0.5 μmol/L SA-B) could also inhibit HSC-T6 cell viability, GSFF at this concentration was not as effective as that at 0.5 μmol/L. We also tested higher doses of GSFF (5 μmol/L and 10 μmol/L), and the results showed no effect on cell viability. Therefore, we concluded that GSFF can be used at an appropriate range of doses to inhibit HSC viability and a higher dose did not mean a more pronounced effect. In addition, SA-B and DG may enhance each other’s effects when combined, so a lower dose of SA-B with DG had a satisfactory effect.
Third, the anti-fibrotic effect of GSFF may be related to an alleviated inflammatory response and reduced ERK phosphorylation. Liver fibrosis is considered an abnormal wound healing response fuelled by a vicious pathogenic circle of hepatocyte necrosis, inflammation and excessive ECM deposition[30]. Though inflammation is not a prerequisite for liver fibrosis[31], in most cases, a persistent inflammatory response is one of the main characteristics of liver fibrosis and participates in HSC activation and collagen synthesis[7,32,33]. GSFF could reduce inflammatory cell infiltration and decrease the release of pro-inflammatory cytokines (IL-1β and TNF-α) as well as NF-κB synthesis in vivo and in vitro. This finding is consistent with that of a previous study showing that SA-B inhibited IL-1β, IL-6, and TNF-α synthesis in a cholestatic liver injury rat model[34]. The ERK cascade is crucial for HSC activation. GSFF inhibited ERK phosphorylation in vivo and in vitro, which is also consistent with the results of a previous study[35]. GSFF also inhibited ERK phosphorylation in HSC-T6 cells stimulated with PDGF-BB in vitro.
In conclusion, the Chinese herbal medicine GSFF alleviates liver fibrosis and HSC-T6 cell activation through inhibiting the inflammatory response and ERK phosphorylation. This study focuses on only one mechanism. Further studies are still needed to analyse the mechanism of GSFF and determine why SA-B in combination with DG has a more pronounced effect than SA-B alone.
Liver fibrosis is a common healthy problem worldwide and there is still a lack of specific medicine. The Chinese herbal medicine,Gan Shen Fu Fang (GSFF) is composed of salvianolic acid B and diammonium glycyrrhizinate. In this study, we observe the effects of GSFF on liver fibrosis in vivo and in vitro in an attempt to provide some hope for the treatment.
During pre-clinical experiment, we found that GSFF could inhibit pseudo-lobule formation in common bile duct-ligated (CBDL) rats. However, the mechanisms remain unclear. Therefore, in this study, we aimed to observe the effects of GSFF on liver fibrosis in vivo and in vitro and determine whether GSFF-mediated alleviation of liver fibrosis is related to inflammation and the ERK signalling pathway.
The present study aimed to observe the effects of GSFF on liver fibrosis in vivo and in vitro and investigate the mechanism from the perspective of the inflammatory response and extracellular signal-regulated kinase (ERK) phosphorylation.
CBDL rats were used for in vivo experiments. Hepatic stellate cells-T6(HSC-T6) cells were used for in vitro experiments. Hematoxylin and eosin and Masson staining, biochemical assays, hydroxyproline assays, enzyme-linked immunoasorbentassay and western blotting were performed to evaluate the degree of liver fibrosis, liver function, the inflammatory response and ERK phosphorylation. The CCK8 assay, immunofluorescence and western blotting were applied to test the effect of GSFF on HSC-T6 cell activation and determine whether GSFF had an effect on ERK phosphorylation in HSC-T6 cells.
GSFF improved liver function and inhibited liver fibrosis in CBDL rats after 3wk of treatment, as demonstrated by histological changes, hydroxyproline assays and collagen I concentrations. GSFF alleviated inflammatory cell infiltration and reduced the synthesis of pro-inflammatory cytokines (tumor necrosis factor-α and interlukin-1β) and NF-κB. In addition, GSFF decreased ERK phosphorylation. In vitro, GSFF inhibited the viability of HSC-T6 cells with and without transforming growth factor β1 stimulation and decreased the synthesis of collagen I. GSFF had the greatest effect at a concentration of 0.5 μmol/L. GSFF inhibited the expression of α-smooth muscle actin, a marker of HSC activation, in HSC-T6 cells. Consistent with the in vivo results, GSFF also inhibited the phosphorylation of ERK and downregulated the expression of NF-κB.
GSFF inhibited liver fibrosis progression in vivo and HSC-T6 cell activation in vitro. These effects may be related to an alleviated inflammatory response and downregulated ERK phosphorylation.
The definite anti-liver fibrosis effect and clear mechanism of GSFF provide hope for the treatment of liver fibrosis.
The authors would like to acknowledge Dr. Zeng-Dun Shi for skilful technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vagholkar K, Yoshioka KS-Editor: Tang JZ L-Editor: MedE-Ma JY E-Editor: Liu JH
| 1. | Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15-S24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 456] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 2. | Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 548] [Cited by in F6Publishing: 526] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 568] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 4. | Böttcher K, Pinzani M. Pathophysiology of liver fibrosis and the methodological barriers to the development of anti-fibrogenic agents. Adv Drug Deliv Rev. 2017;121:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Hou W, Syn WK. Role of Metabolism in Hepatic Stellate Cell Activation and Fibrogenesis. Front Cell Dev Biol. 2018;6:150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20:2515-2532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 247] [Cited by in F6Publishing: 227] [Article Influence: 22.7] [Reference Citation Analysis (3)] |
| 7. | Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 548] [Cited by in F6Publishing: 635] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 8. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1361] [Cited by in F6Publishing: 1440] [Article Influence: 84.7] [Reference Citation Analysis (1)] |
| 9. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1221] [Cited by in F6Publishing: 1612] [Article Influence: 230.3] [Reference Citation Analysis (0)] |
| 10. | Wu KC, Ho YL, Kuo YH, Huang SS, Huang GJ, Chang YS. Hepatoprotective Effect of Ugonin M, A Helminthostachyszeylanica Constituent, on Acetaminophen-Induced Acute Liver Injury in Mice. Molecules. 2018;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Zhang L, Xiao X, Arnold PR, Li XC. Transcriptional and epigenetic regulation of immune tolerance: roles of the NF-κB family members. Cell Mol Immunol. 2019;16:315-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Foglia B, Cannito S, Bocca C, Parola M, Novo E. ERK Pathway in Activated, Myofibroblast-Like, Hepatic Stellate Cells: A Critical Signaling Crossroad Sustaining Liver Fibrosis. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Kocabayoglu P, Lade A, Lee YA, Dragomir AC, Sun X, Fiel MI, Thung S, Aloman C, Soriano P, Hoshida Y, Friedman SL. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63:141-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Novo E, Marra F, Zamara E, Valfrè di Bonzo L, Caligiuri A, Cannito S, Antonaci C, Colombatto S, Pinzani M, Parola M. Dose dependent and divergent effects of superoxide anion on cell death, proliferation, and migration of activated human hepatic stellate cells. Gut. 2006;55:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Ding ZY, Jin GN, Liang HF, Wang W, Chen WX, Datta PK, Zhang MZ, Zhang B, Chen XP. Transforming growth factor β induces expression of connective tissue growth factor in hepatic progenitor cells through Smad independent signaling. Cell Signal. 2013;25:1981-1992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Bocca C, Novo E, Miglietta A, Parola M. Angiogenesis and Fibrogenesis in Chronic Liver Diseases. Cell Mol Gastroenterol Hepatol. 2015;1:477-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Du QH, Li WH, Xu Y, Han L, Jia X, Zhao T. The Protective Effect of Gan Shen Fu Fang on Liver Endothelial Cells in Common Bile Ductligated Rats. World J TraditChinMed. 2017;3:5. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Du QH, Han L, Jiang JJ, Xu Y, Li WH, Li PT, Wang XY, Jia X. Glytan decreases portal pressure via mesentery vasoconstriction in portal hypertensive rats. World J Gastroenterol. 2014;20:16674-16682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Durairajan SSK, Chirasani VR, Shetty SG, Iyaswamy A, Malampati S, Song J, Liu L, Huang J, Senapati S, Li M. Decrease in the Generation of Amyloid-β Due to Salvianolic Acid B by Modulating BACE1 Activity. Curr Alzheimer Res. 2017;14:1229-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Yan XF, Zhao P, Ma DY, Jiang YL, Luo JJ, Liu L, Wang XL. Salvianolic acid B protects hepatocytes from H2O2 injury by stabilizing the lysosomal membrane. World J Gastroenterol. 2017;23:5333-5344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, Weiskirchen R. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp. 2015;96:52438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 22. | Van Campenhout S, Van Vlierberghe H, Devisscher L. Common Bile Duct Ligation as Model for Secondary Biliary Cirrhosis. Methods Mol Biol. 2019;1981:237-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Yokota S, Ono Y, Nakao T, Zhang P, Michalopoulos GK, Khan Z. Partial Bile Duct Ligation in the Mouse: A Controlled Model of Localized Obstructive Cholestasis. J Vis Exp. 2018;133:56930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Wang R, Yu XY, Guo ZY, Wang YJ, Wu Y, Yuan YF. Inhibitory effects of salvianolic acid B on CCl(4)-induced hepatic fibrosis through regulating NF-κB/IκBα signaling. J Ethnopharmacol. 2012;144:592-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 750] [Cited by in F6Publishing: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 26. | Nishio T, Hu R, Koyama Y, Liang S, Rosenthal SB, Yamamoto G, Karin D, Baglieri J, Ma HY, Xu J, Liu X, Dhar D, Iwaisako K, Taura K, Brenner DA, Kisseleva T. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J Hepatol. 2019;71:573-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Li D, He L, Guo H, Chen H, Shan H. Targeting activated hepatic stellate cells (aHSCs) for liver fibrosis imaging. EJNMMI Res. 2015;5:71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 29. | Zhang W, Ping J, Zhou Y, Chen G, Xu L. Salvianolic Acid B Inhibits Activation of Human Primary Hepatic Stellate Cells Through Downregulation of the Myocyte Enhancer Factor 2 Signaling Pathway. Front Pharmacol. 2019;10:322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Pinzani M. Pathophysiology of Liver Fibrosis. Dig Dis. 2015;33:492-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Martin P, D'Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 399] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 32. | Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, Liepelt A, Lefebvre E, Luedde T, Hellerbrand C, Weiskirchen R, Longerich T, Costa IG, Anstee QM, Trautwein C, Tacke F. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 338] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 33. | Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 900] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 34. | Li S, Wang R, Wu B, Wang Y, Song F, Gu Y, Yuan Y. Salvianolic acid B protects against ANIT-induced cholestatic liver injury through regulating bile acid transporters and enzymes, and NF-κB/IκB and MAPK pathways. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1169-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Lv Z, Song Y, Xue D, Zhang W, Cheng Y, Xu L. Effect of salvianolic-acid B on inhibiting MAPK signaling induced by transforming growth factor-β1 in activated rat hepatic stellate cells. J Ethnopharmacol. 2010;132:384-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |