Published online Apr 28, 2020. doi: 10.3748/wjg.v26.i16.1901
Peer-review started: December 31, 2019
First decision: January 13, 2020
Revised: March 26, 2020
Accepted: April 4, 2020
Article in press: April 4, 2020
Published online: April 28, 2020
Processing time: 118 Days and 21.5 Hours
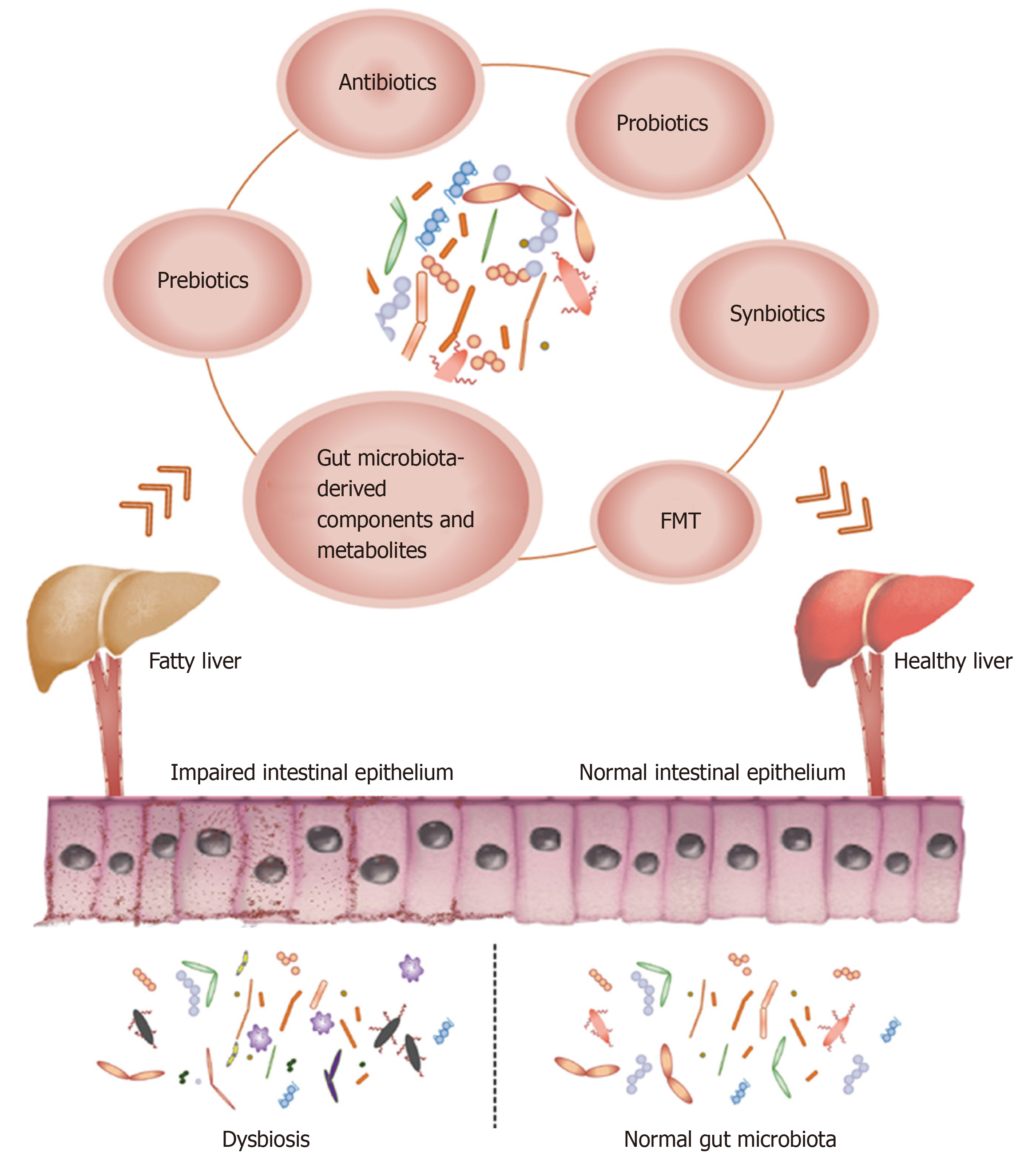
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent metabolic disorder with steadily increasing incidence rates worldwide, especially in the West. There are no drugs available at present to treat NAFLD, and the primary therapeutic options include weight loss and the combination of healthy diet and exercise. Therefore, novel interventions are required that can target the underlying risk factors. Gut microbiota is an “invisible organ” of the human body and vital for normal metabolism and immuno-modulation. The number and diversity of microbes differ across the gastrointestinal tract from the mouth to the anus, and is most abundant in the intestine. Since dysregulated gut microbiota is an underlying pathological factor of NAFLD, it is a viable therapeutic target that can be modulated by antibiotics, probiotics, prebiotics, synbiotics, fecal microbiota transplantation, and microbial metabolites. In this review, we summarize the most recent advances in gut microbiota-targeted therapies against NAFLD in clinical and experimental studies, and critically evaluate novel targets and strategies for treating NAFLD.
Core tip: Non-alcoholic fatty liver disease is a highly prevalent metabolic disease worldwide. In this review, we summarize the most recent advances in gut microbiota-targeted therapies against non-alcoholic fatty liver disease, including antibiotics, probiotics, prebiotics, synbiotics, fecal microbiota transplantation, and the gut microbiota-derived components and metabolites.
- Citation: Chen HT, Huang HL, Li YQ, Xu HM, Zhou YJ. Therapeutic advances in non-alcoholic fatty liver disease: A microbiota-centered view. World J Gastroenterol 2020; 26(16): 1901-1911
- URL: https://www.wjgnet.com/1007-9327/full/v26/i16/1901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i16.1901
The liver is the largest organ in our body, with vital functions in digestion, energy storage, and detoxification. Fatty liver disease is the most common hepato-pathological condition characterized by excessive fat accumulation in the liver. Based on the etiology, fatty liver disease can be classified into the alcoholic and non-alcoholic types. As the name indicates, alcoholic fatty liver disease is the result of alcohol overconsumption. Ethanol metabolism in the liver produces fatty acids which steadily accumulate within the liver cells, along with acetaldehyde and free radicals that also have deleterious effects on the liver and other organs[1]. Non-alcoholic fatty liver disease (NAFLD) is caused by multiple factors including poor diet, insulin resistance, and other metabolic disturbances[2]. In addition, lifestyle-related factors like sleep shortage, irregular food intake, sedentary habits, and excessive weight gain are also risk factors for NAFLD[3]. It can be further sub-divided into the fatty liver without inflammation and nonalcoholic steatohepatitis (NASH) types. The latter frequently progresses to fibrosis, advanced cirrhosis, hepatocellular carcinoma (HCC), and even death[4]. NAFLD is in fact the most rapidly increasing underlying condition requiring liver transplantation[5], and can be considered a hepatic manifestation of the metabolic syndrome. Several clinical trials are underway to develop novel therapies against NAFLD[6], since the current treatments focusing on lifestyle modifications have been largely approved. Mediterranean diet and physical activity for instance have been shown to prevent the onset of NAFLD[7,8]. The ultimate goal of NAFLD treatment is to inhibit fibrotic development that can eventually lead to cirrhosis and HCC[6]. However, there are no Food and Drug Administration approved drugs at present for treating this condition[9].
The gut microbiota is considered by many as a “metabolic organ” that plays a vital role in host metabolism and liver function[10]. In addition to the classic “two-hit” theory or the updated “multiple hit” model[11], intestinal dysbiosis is also a causative factor of NAFLD, and promotes its progression by modulating host energy metabolism, insulin sensitivity, immune response, and inflammation[12]. The pathophysiological relationship between the gut microbiota and NAFLD is complex and involves diverse immunological and metabolic pathways. For instance, impaired intestinal permeability in mice lacking the junctional adhesion molecule A protein (Jam1) or Muc2 increases the risk of liver inflammation when the animals are fed a high-fat diet (HFD)[13,14]. Furthermore, the microbiota from adult NAFLD patients exhibits differences in carbon and amino acid metabolism[15]. NAFLD is also associated with increased serum TMAO levels and hepatic bile acid (BA) synthesis[16] and less production of phosphatidylcholine[17]. The pathological roles of various bacterial metabolites and microbiota-generated secondary BA in NAFLD have been unearthed in recent years[18]. These metabolites can trigger metabolic dysfunction and contribute to NAFLD development and progression by targeting relevant pathways. The gut microbiota also diversifies the repertoire of host BAs by modulating its metabolism, thereby regulating pathways mediated by BA receptors such as farnesoid X receptor (FXR) and TGR5[19,20].
Therefore, researchers are increasingly focusing on the gut microbiota as a new therapeutic target for NAFLD, and have developed various treatment modalities including antibiotics, probiotics, prebiotics, synbiotics, fecal microbiota trans-plantation (FMT), gut microbiota-derived components, and metabolites (Figure 1). In this review, we summarize the most recent advances in gut microbiota-targeted therapies against NAFLD in clinical and experimental studies, and critically evaluate novel targets and strategies for treating NAFLD.
Antibiotics can eliminate harmful microbiota, and their efficacy has been confirmed in various liver diseases[21-27]. Since the 1950s[28,29], neomycin, metronidazole, rifaximin, and polymyxin B have been used extensively for treating cirrhosis and hepatic encephalopathy. In addition, concurrent polymyxin B and neomycin use prevented lipid accumulation in the liver by altering the gut microbiota[30]. Gangarapu et al[31] found that short-term administration of antibiotics improved the clinical symptoms in NAFLD/NASH patients by lowering circulating endotoxins as well as serum transaminases. Consistent with this, another study[32] reported a significant reduction in the levels of transaminase and NAFLD-liver fat score after rifaximin treatment. However, in a recent clinical trial conducted by Ponziani et al[33], rifaximin showed little therapeutic effects against NASH. This discrepancy could be the result of low drug dose, short duration of the treatment, and small sample size. Antibiotic-induced changes in the gut microbiota can provide valuable insights into its therapeutic utility in various diseases. Specific antibiotics can positively affect the gut microbiota by promoting the growth of beneficial gut bacteria like Bifidobacteria and Lactobacilli. While short-term antibiotic treatment may have a therapeutic effect, long-term application can lead to the emergence of bacterial resistance, thereby limiting the efficacy of the drug and increasing the risk of secondary infections. Therefore, chronic antibiotic use is not encouraged since they can affect the beneficial gut bacteria and cause intestinal dysbiosis[34].
Probiotics are non-pathogenic microbes that alleviate gut disorders by restoring the normal microbiota, and provide overall health benefits to the host[35]. These beneficial bacteria can reduce lipid deposition, endotoxemia, oxidative stress, and inflammation by regulating the expression levels of TNF-α, NF-κB, and collagen[35]. Probiotic strains for therapeutic applications are selected on the basis of safety, functionality, and technical requirements[36]. For instance, some Streptococcus, Lactobacillus, and Bifidobacteria strains can regulate the mucosa-motivated immune system and gastrointestinal inflammation, and promote the growth and survival of gut epithelial cells[37].
Probiotics have shown significant therapeutic effects on the murine fatty liver model as well. Administrating probiotics to mice fed an HFD significantly slowed the progression of hepatic steatosis and fibrosis[35]. However, most studies on NAFLD rodent models have been aimed at preventing, rather than treating, diet-induced liver disease[38]. Clinical trials on NAFLD patients have shown that Lactobacillus, Streptococcus, and Bifidobacterium strains play an ameliorative role by restoring the levels of the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT)[35]. For instance, the intra-hepatic levels of AST and ALT increased significantly in NAFLD patients following 3 mo of treatment with Lactobacillus bulgaricus (L. bulgaricus) and Streptococcus thermophilus (S. thermophiles)[39]. MIYAIRI 588, a probiotic Clostridium butyricum strain originally from Japan and used widely in Asia, prevented fatty degeneration from progressing to liver cancer in a rat NAFLD model[40,41]. In addition, co-administration of several probiotic strains, such as the VSL3 formulation including eight probiotic bacterial strains [S. thermophiles, Bifidobacterium breve, Bifidobacterium longum (B. longum), Bifidobacterium infantis, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, and L. bulgaricus] resulted in greater therapeutic effects compared to any single strain[42-44]. A randomized controlled trial conducted on overweight children with NAFLD showed significant improvement in the fatty liver condition and BMI following treatment with VSL3. Subsequent studies indicated that the increase in total and active GLP-1 as well as decrease in the plasma levels of S-nitrosothiols, malondialdehyde, and 4-hydroxynonenal was the potential mechanisms underlying the therapeutic effects of VSL3[45,46]. Furthermore, VSL3 can alleviate chronic liver diseases by protecting the intestinal barrier and reducing endotoxemia and oxidative/nitrosative stress[46,47].
However, probiotics are primarily derived from bacteria, which raises concerns of biosafety. A few probiotics derived from yeast (e.g., Saccharomyces boulardii) have shown encouraging effects, especially when combined with traditional bacterial probiotics[48]. Further research is needed to optimize the efficacy, safety, and sustainability of probiotics for treating NAFLD.
The International Scientific Association for Probiotics and Prebiotics defines prebiotics as substrates that are broken down by host microorganisms into metabolites[22] that promote the growth of beneficial bacteria[49]. Prebiotic feeding is an effective adjuvant therapy for liver diseases, which improves the symptoms by restoring gut microbiota[10,50]. Oligofructose, a mixture of nondigestible fermentable dietary fiber[51], reduced liver oxidative stress and inflammation by improving intestinal permeability and tight junction integrity. Prebiotics stimulated the growth of Bifidobacteria and normalized plasma endotoxin levels, which improved glucose tolerance and subsequently resulted in weight loss in obese individuals[52]. Lactulose is another prebiotic that promotes the growth of Bifidobacteria, Lactobacillus, and Gram-positive bacteria and inhibits the endotoxemic Gram-negative bacteria[53]. HFD-fed obese mice that were administered lactulose for 6 wk showed reduced inflammation and liver damage, which correlated to decreased circulating levels of lipopolysaccharides[54]. In addition, the fungal prebiotic chitin-glucan can also limit weight gain, glucose intolerance, liver triglyceride accumulation, and fasting hyperglycemia by modulating the gut microbiota[55].
The beneficial effects of prebiotics on NAFLD can be attributed to reduced de novo lipogenesis, weight and fat loss, improved blood glucose control, restored gut microbiota, and lower inflammation[56]. Clinical trials have also demonstrated therapeutic effects of prebiotics on NAFLD/NASH progression via modulation of glucose homeostasis and lipid metabolism[57]. In conclusion, prebiotics are a highly suitable therapeutic tool against NAFLD.
Synbiotics are the combination of probiotics and prebiotics. NASH patients treated with Bifidobacterium and fructo-oligosaccharides (FOS) for 6 mo showed significantly lower serum ALT and AST levels compared to the placebo group[58], indicating the potential advantage of using synbiotics against liver diseases. Another study showed that synbiotic supplementation with seven probiotic strains (Lactobacillus casei, L. bulgaricus, Lactobacillus rhamnosus, Lactobacillus acidophilus, Bifidobacterium breve, B. longum, and S. thermophilus) and FOS for 28 wk, along with healthy lifestyle modifications, was more beneficial (in terms of reduced inflammation and BMI) to NAFLD patients compared to lifestyle changes alone[59]. Malaguarnera et al[60] reported that co-administering B. longum and FOS for 24 wk combined with a healthy lifestyle significantly decreased NASH activity index and hepatic fat accumulation. Likewise, Safavi et al[61] found that long-term synbiotic treatment significantly decreased serum lipid levels in obese children. A meta-analysis of 15 randomized controlled trials including a total of 782 NAFLD patients showed that synbiotics markedly attenuated liver steatosis, ALT, AST, high-density lipoprotein, low-density lipoprotein, triglyceride and cholesterol levels, TNF-α expression, the degree of liver stiffness, and homeostasis model assessment-insulin resistance[62].
FMT involves transferring functional microbiomes from the feces of healthy individuals to the gastrointestinal tract of patients with intestinal dysbiosis. It was introduced by Chinese medical and herbal practitioners for treating severe diarrhea and food poisoning[63]. FMT is an effective therapeutic option for recurrent Clostridium difficile infection, as well as liver and metabolic diseases associated with intestinal microbiota dysbiosis. The clinical studies conducted so far on microbiota-targeting strategies, including FMT, in NAFLD patients are summarized in Table 1.
| NCT number | Condition(s) | Intervention | Phase | Status | Country |
| NCT01355575 | NAFLD | Antibiotics | Phase 4 | Terminated | United Kingdom |
| NCT02329405 | NAFLD | Antibiotics | Phase 4 | Completed | Finland |
| NCT01759628 | NAFLD | Antibiotics | Phase 2 | Completed | Iran |
| NCT01712711 | NAFLD | Antibiotics | Phase 2 | Completed | Iran |
| NCT01654549 | NAFLD | Antibiotics | Phase 2 | Completed | Iran |
| NCT01876108 | Fatty liver | Antibiotics | Phase 2 | Completed | Iran |
| NCT02510599 | NASH | Antibiotics | Phase 2 | Completed | United States |
| NCT00068094 | Fatty liver | Probiotics | Phase 1/Phase 2 | Terminated | United States |
| NCT02972567 | Metabolic syndrome/NAFLD | Probiotics | Phase 2 | Unknown status1 | Spain |
| NCT03511365 | NAFLD | Probiotics | Phase 1/Phase 2 | Terminated | United States |
| NCT03585413 | Obesity/NAFLD | Probiotics | Phase 3 | Recruiting | Germany |
| NCT04175392 | Fatty liver disease | Probiotics | Phase 1/Phase 2 | Not yet recruiting | United States |
| NCT02530138 | NASH | Synbiotics | Phase 2/Phase 3 | Unknown status1 | Iran |
| NCT01791959 | NASH | Synbiotics | Phase 2/Phase 3 | Completed | Iran |
| NCT02496390 | Diabetes mellitus/NAFLD | FMT | Phase 1/Phase 2 | Completed | Canada |
| NCT02530385 | Obesity/NAFLD | FMT | Phase 1/Phase 2 | Completed | United States |
| NCT02741518 | Obesity/NAFLD | FMT | Phase 1/Phase 2 | Active, not recruiting | United States |
| NCT02970877 | Obesity/NAFLD | FMT | Phase 2 | Recruiting | Canada |
| NCT02050607 | Metabolic syndrome/NAFLD | FMT | Phase 3 | Unknown status1 | Italy |
| NCT02862249 | Cirrhosis | FMT | Phase 3 | Recruiting | United Kingdom |
| NCT03014505 | Cirrhosis | FMT | Phase 1/Phase 2 | Unknown status1 | China |
Studies show[64,65] that transplanting the gut microbiota from lean or obese mice induced phenotypes similar to that of the host, with the microbiota of lean donors significantly reducing adiposity in the obese mice. However, Fischer et al[66] observed no improvement in the BMI of Clostridium difficile infection patients within 12 mo of a single FMT, regardless of the donor BMI. In contrast, overweight patients with metabolic syndrome showed a significant improvement in hepatic (119%) and peripheral (176%) insulin sensitivity 6 wk after receiving microbiota from lean healthy controls compared to the autologous microbiota[67]. Several studies have demonstrated the therapeutic effects of FMT on type 2 diabetes and ulcerative colitis patients[68-72], which were associated with restored healthy microbiota, normalized blood lipid levels, and improved insulin resistance.
Based on these reports, we can surmise that FMT is a potential therapeutic option for NAFLD and NASH as well. To determine the role of the gut microbiota in NAFLD development, Le Roy et al[73] transplanted the feces from HFD responder and non-responder mice into germ-free recipients. The mice that received microbiota from the responder group developed steatosis and showed a high abundance of Barnesiella and Roseburia in the intestine, whereas microbiota from the non-responder mice markedly increased the abundance of Allobaculum in the recipients. Furthermore, an 8-wk FMT intervention[74] significantly restored the disordered gut microbiota in HFD-induced NASH mouse models by increasing the abundance of beneficial bacteria such as Christensen and Lactobacillus. It also alleviated endotoxemia, liver steatosis, necrosis, and intra-hepatic inflammation compared to the untreated controls. Consistent with this, metabolic syndrome patients transplanted with the gut microbes of healthy individuals showed increased butyrate production and improved insulin sensitivity[75], which could be attributed to the higher abundance of beneficial bacteria in the lower gut.
Fecal matter can be implanted through nasogastric tubes, nasojejunostomy tubes, upper gastrointestinal endoscopy (gastroduodenoscopy), colonoscopy, or retention enema, and the outcomes of these methods differ significantly. In addition, the heterogeneity of donor fecal matter also influences the therapeutic effect. FMT is also associated with the risk of unpredictable infections from the transplanted microorganisms under certain circumstances. Finally, the stability of foreign bacteria into the host gut is limited, which can reduce their long-term survival and therapeutic effects[76]. Therefore, further clinical trials are warranted to confirm the therapeutic benefit of this strategy.
Studies[77-84] show that the interaction between gut microbiota and their hosts is mediated by various metabolites that are secreted, degraded, or modified by the former, such as short-chain and long-chain fatty acids, amino acids, bile acids, vitamins, and polysaccharides. These metabolites form an intricate signaling network that affects host metabolism and prevents the growth of pathogenic bacteria, and therefore can be utilized to restore the gut microbiota and supplement the effects of FMT or probiotics[85].
Short chain fatty acids (SCFA) supplementation have shown ameliorative effects in cancer[86,87], metabolic diseases[88-90], and other diseases. SCFA is produced during the fermentation of dietary fiber in the gut, and can activate G protein coupled receptor and lower histone deacetylase activity. It is also a fuel for gut epithelial cells and regulates multiple metabolic pathways in the intestine[91]. SCFA administration in metabolic diseases modulates immune homeostasis, gut hormone secretion, inflammatory response, gut barrier, and other functions[92-95].
Bile acid (BA) is a cholesterol derivative that is synthesized and conjugated in the liver. It plays a central role in digestion by emulsifying dietary fats and promoting the absorption of lipids and vitamins in the small intestine (mainly the ileum), which affects hepatic lipid accumulation and inflammation. Thus, BA is a critical signaling molecule that functionally connects the intestine and liver. The BA receptor (BAR), also known as FXR, is highly expressed in the liver and intestine and regulates the synthesis of bile acids through a feedback mechanism. A recent study identified TGR5 as another major BAR in the liver[96]. The underlying mechanisms remain to be elucidated in order to determine whether FXR agonistic or antagonistic effects are beneficial to NAFLD. Amino acid catabolites play a regulatory role in NAFLD by influencing intestinal epithelial barrier and have therapeutic effects on liver function[96]. Indole propionic acid and other indole-like molecules maintain the integrity of intestinal epithelial barrier[97] and control inflammation, and can directly act on hepatocytes and liver immune cells[98].
Gut microbiota-derived metabolites can overcome the major disadvantage of colonization resistance associated with probiotics and FMT. However, metabolite therapy also has several limitations that ought to be considered[85]. First, the endogenous gut microbiota and the exogenous metabolites may interact unpredictably, which can aggravate the intestinal dysbiosis or even alter the gut microbiota to produce harmful metabolites. Second, the sudden change in the intestinal levels of the supplemented metabolites may also disrupt the feedback loops of the endogenous metabolites. Long-term supplementation of metabolites can even lead to the emergence of host or bacterial resistance, and thus alter the therapeutic target. Third, the low level of some metabolites in the feces may not truly reflect the status in the intestine, and the suboptimal absorption of oral metabolites in the proximal gastrointestinal tract would decrease their effects on the distal small intestine and colon. In addition, the long-term and systemic effects of these metabolites are unknown and need to be elucidated through detailed pharma-cokinetics and pharmacodynamics studies. Finally, bacterial metabolites have very complex chemical structures and some are even volatile, which makes laboratory synthesis technically challenging[85]. Taken together, the clinical application of bacterial metabolites will have to be supported by strong experimental foundations.
NAFLD is a common chronic liver disease that can progress to cirrhosis and HCC, and the prevalence of NAFLD/NASH is increasing globally. The advent of 16S high-throughput sequencing has increased the potential for microbiota-targeted NAFLD/NASH treatment. Apart from bacteria, the intestinal microbiome includes fungi, viruses, and archaeabacteria, which are associated with various liver diseases. Therefore, it is logical to target the gut–liver axis, especially the microbiota, in order to alleviate the symptoms of NAFLD. Although conventional antibiotics can modulate NAFLD symptoms, their clinical use is largely limited due to their side effects and the emergence and prevalence of bacterial resistance. Probiotics, prebiotics, and synbiotics are safe and effective alternatives to conventional antibiotics for treating NAFLD. In addition, FMT is also a promising strategy that can reverse the intestinal dysbiosis associated with NAFLD. Novel therapies involving gut microbiota-derived components and metabolites are increasingly being developed for their unique advantages. The next generation microbiota-targeted therapies against NAFLD include genetically engineered microbiota and recombinant metabolites. Furthermore, the genomes of NAFLD patients and possible genetic determinants of therapeutic responses should also be explored to develop more personalized therapies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee HC, Musumeci G S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Trovato FM, Martines GF, Catalano D, Musumeci G, Pirri C, Trovato GM. Echocardiography and NAFLD (non-alcoholic fatty liver disease). Int J Cardiol. 2016;221:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Trovato FM, Castrogiovanni P, Szychlinska MA, Purrello F, Musumeci G. Early effects of high-fat diet, extra-virgin olive oil and vitamin D in a sedentary rat model of non-alcoholic fatty liver disease. Histol Histopathol. 2018;33:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G, Trovato GM. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Cholankeril G, Patel R, Khurana S, Satapathy SK. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Current knowledge and implications for management. World J Hepatol. 2017;9:533-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (3)] |
| 5. | Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol. 2017;9:715-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 504] [Article Influence: 63.0] [Reference Citation Analysis (17)] |
| 6. | Suk KT, Kim DJ. Gut microbiota: novel therapeutic target for nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Trovato FM, Catalano D, Musumeci G, Trovato GM. 4Ps medicine of the fatty liver: the research model of predictive, preventive, personalized and participatory medicine-recommendations for facing obesity, fatty liver and fibrosis epidemics. EPMA J. 2014;5:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Trovato FM, Castrogiovanni P, Malatino L, Musumeci G. Nonalcoholic fatty liver disease (NAFLD) prevention: role of Mediterranean diet and physical activity. Hepatobiliary Surg Nutr. 2019;8:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Wong VW, Singal AK. Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis. Transl Gastroenterol Hepatol. 2019;4:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Han R, Ma J, Li H. Mechanistic and therapeutic advances in non-alcoholic fatty liver disease by targeting the gut microbiota. Front Med. 2018;12:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Ding Y, Yanagi K, Cheng C, Alaniz RC, Lee K, Jayaraman A. Interactions between gut microbiota and non-alcoholic liver disease: The role of microbiota-derived metabolites. Pharmacol Res. 2019;141:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Zhou D, Fan JG. Microbial metabolites in non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:2019-2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 13. | Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, Stärkel P, Belzer C, Hellerbrand C, Tsukamoto H, Ho SB, Schnabl B. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 14. | Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, Wu P, Liu X, Yu Y, Farris AB, Nusrat A, Parkos CA, Anania FA. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016;151:733-746.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 15. | Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2019;30:607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 16. | Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, Fung S, Fischer SE, McGilvray IG, Allard JP. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS One. 2016;11:e0151829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (1)] |
| 17. | Arendt BM, Ma DW, Simons B, Noureldin SA, Therapondos G, Guindi M, Sherman M, Allard JP. Nonalcoholic fatty liver disease is associated with lower hepatic and erythrocyte ratios of phosphatidylcholine to phosphatidylethanolamine. Appl Physiol Nutr Metab. 2013;38:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 18. | Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 19. | Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology. 2017;65:350-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 450] [Article Influence: 56.3] [Reference Citation Analysis (1)] |
| 20. | Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 358] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 21. | Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53:362-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 505] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 22. | Cho MS, Kim SY, Suk KT, Kim BY. Modulation of gut microbiome in nonalcoholic fatty liver disease: pro-, pre-, syn-, and antibiotics. J Microbiol. 2018;56:855-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 537] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 24. | Ma J, Zhou Q, Li H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 25. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1546] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 26. | Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci. 2019;76:1541-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 337] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 27. | Sidhu M, van der Poorten D. The gut microbiome. Aust Fam Physician. 2017;46:206-211. [PubMed] |
| 28. | Dawson AM, Mclaren J, Sherlock S. Neomycin in the treatment of hepatic coma. Lancet. 1957;273:1262-1268. [PubMed] |
| 29. | Sharma P, Sharma BC. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:2423-2424; author reply 2424-2425. [PubMed] |
| 30. | Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 400] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 31. | Gangarapu V, Ince AT, Baysal B, Kayar Y, Kılıç U, Gök Ö, Uysal Ö, Şenturk H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Abdel-Razik A, Mousa N, Shabana W, Refaey M, Elzehery R, Elhelaly R, Zalata K, Abdelsalam M, Eldeeb AA, Awad M, Elgamal A, Attia A, El-Wakeel N, Eldars W. Rifaximin in nonalcoholic fatty liver disease: hit multiple targets with a single shot. Eur J Gastroenterol Hepatol. 2018;30:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Ponziani FR, Zocco MA, D'Aversa F, Pompili M, Gasbarrini A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol. 2017;23:4491-4499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 34. | Singh R, Sripada L, Singh R. Side effects of antibiotics during bacterial infection: mitochondria, the main target in host cell. Mitochondrion. 2014;16:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Xie C, Halegoua-DeMarzio D. Role of Probiotics in Non-alcoholic Fatty Liver Disease: Does Gut Microbiota Matter? Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Markowiak P, Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1200] [Cited by in RCA: 1220] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 37. | Paolella G, Mandato C, Pierri L, Poeta M, Di Stasi M, Vajro P. Gut-liver axis and probiotics: their role in non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:15518-15531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (3)] |
| 38. | Meroni M, Longo M, Dongiovanni P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090-1095. [PubMed] |
| 40. | Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 41. | Seo M, Inoue I, Tanaka M, Matsuda N, Nakano T, Awata T, Katayama S, Alpers DH, Komoda T. Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig Dis Sci. 2013;58:3534-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Mora D, Filardi R, Arioli S, Boeren S, Aalvink S, de Vos WM. Development of omics-based protocols for the microbiological characterization of multi-strain formulations marketed as probiotics: the case of VSL#3. Microb Biotechnol. 2019;12:1371-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 43. | Salminen S, Nybom S, Meriluoto J, Collado MC, Vesterlund S, El-Nezami H. Interaction of probiotics and pathogens--benefits to human health? Curr Opin Biotechnol. 2010;21:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics--A comparison of functionality and efficacy. Int J Food Microbiol. 2004;96:219-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 45. | Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 340] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 46. | Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 339] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 47. | Meroni M, Longo M, Dongiovanni P. Alcohol or Gut Microbiota: Who Is the Guilty? Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 48. | Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol. 2012;5:111-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 49. | Vallianou N, Stratigou T, Christodoulatos GS, Dalamaga M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr Obes Rep. 2019;8:317-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 50. | Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 649] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 51. | Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 2006;55:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 296] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 52. | Koopman N, Molinaro A, Nieuwdorp M, Holleboom AG. Review article: can bugs be drugs? The potential of probiotics and prebiotics as treatment for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;50:628-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 54. | Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 588] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 55. | Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2012;23:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 56. | Parnell JA, Raman M, Rioux KP, Reimer RA. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012;32:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 57. | Fernández-Musoles R, García Tejedor A, Laparra JM. Immunonutritional contribution of gut microbiota to fatty liver disease. Nutr Hosp. 2020;37:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Jafarpour D, Shekarforoush SS, Ghaisari HR, Nazifi S, Sajedianfard J, Eskandari MH. Protective effects of synbiotic diets of Bacillus coagulans, Lactobacillus plantarum and inulin against acute cadmium toxicity in rats. BMC Complement Altern Med. 2017;17:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 60. | Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, Galvano F. Bifidobacterium longum with fructo-oligosaccharides in patients with non-alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 327] [Article Influence: 25.2] [Reference Citation Analysis (1)] |
| 61. | Safavi M, Farajian S, Kelishadi R, Mirlohi M, Hashemipour M. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: a randomized triple-masked controlled trial. Int J Food Sci Nutr. 2013;64:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Liu L, Li P, Liu Y, Zhang Y. Efficacy of Probiotics and Synbiotics in Patients with Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Dig Dis Sci. 2019;64:3402-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 63. | Choi HH, Cho YS. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin Endosc. 2016;49:257-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 64. | Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 2710] [Article Influence: 225.8] [Reference Citation Analysis (0)] |
| 65. | Walker AW, Parkhill J. Microbiology. Fighting obesity with bacteria. Science. 2013;341:1069-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Fischer M, Sipe B, Torbeck M, Xu H, Kassam Z, Allegretti J. Does Fecal Microbiota Transplantation from an Obese Donor Lead to Weight Gain? A Case Series of 70 Recipients. Gastroenterology. 2017;152:S1004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-916.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2000] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 68. | Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 69. | Cai TT, Ye XL, Yong HJ, Song B, Zheng XL, Cui BT, Zhang FM, Lu YB, Miao H, Ding DF. Fecal microbiota transplantation relieve painful diabetic neuropathy: A case report. Medicine (Baltimore). 2018;97:e13543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, Herrema H, Ackermans M, Serlie MJM, de Brauw M, Levels JHM, Sales A, Gerdes VE, Ståhlman M, Schimmel AWM, Dallinga-Thie G, Bergman JJ, Holleman F, Hoekstra JBL, Groen A, Bäckhed F, Nieuwdorp M. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69:502-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 71. | He Z, Cui BT, Zhang T, Li P, Long CY, Ji GZ, Zhang FM. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: The first report. World J Gastroenterol. 2017;23:3565-3568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 177] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (5)] |
| 72. | Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611-619.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 669] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 73. | Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 709] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 74. | Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 288] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 75. | Bajaj JS, Fagan A, Sikaroodi M, White MB, Sterling RK, Gilles H, Heuman D, Stravitz RT, Matherly SC, Siddiqui MS, Puri P, Sanyal AJ, Luketic V, John B, Fuchs M, Ahluwalia V, Gillevet PM. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl. 2017;23:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 76. | Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102-109.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1074] [Article Influence: 107.4] [Reference Citation Analysis (1)] |
| 77. | Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 664] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 78. | Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 1581] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 79. | Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling - mechanisms and research needs. Nat Rev Endocrinol. 2019;15:701-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 80. | Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 4124] [Article Influence: 458.2] [Reference Citation Analysis (0)] |
| 81. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 2218] [Article Influence: 277.3] [Reference Citation Analysis (1)] |
| 82. | Levy M, Blacher E, Elinav E. Microbiome, metabolites and host immunity. Curr Opin Microbiol. 2017;35:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 326] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 83. | Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 471] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 84. | Lustri BC, Sperandio V, Moreira CG. Bacterial Chat: Intestinal Metabolites and Signals in Host-Microbiota-Pathogen Interactions. Infect Immun. 2017;85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 85. | Suez J, Elinav E. The path towards microbiome-based metabolite treatment. Nat Microbiol. 2017;2:17075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 86. | McIntyre A, Gibson PR, Young GP. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993;34:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 383] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 87. | Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 371] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 88. | Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1337] [Cited by in RCA: 1556] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 89. | Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 920] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 90. | De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1600] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 91. | Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1334] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 92. | Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204-12209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1664] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 93. | Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2568] [Cited by in RCA: 2861] [Article Influence: 190.7] [Reference Citation Analysis (0)] |
| 94. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 3901] [Article Influence: 325.1] [Reference Citation Analysis (0)] |
| 95. | Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 769] [Article Influence: 69.9] [Reference Citation Analysis (1)] |
| 96. | Kim H, Fang S. Crosstalk between FXR and TGR5 controls glucagon-like peptide 1 secretion to maintain glycemic homeostasis. Lab Anim Res. 2018;34:140-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 97. | Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol. 2018;8:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 752] [Cited by in RCA: 834] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 98. | Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 762] [Article Influence: 84.7] [Reference Citation Analysis (0)] |