Copyright
©The Author(s) 2020.
World J Gastroenterol. Apr 7, 2020; 26(13): 1525-1539
Published online Apr 7, 2020. doi: 10.3748/wjg.v26.i13.1525
Published online Apr 7, 2020. doi: 10.3748/wjg.v26.i13.1525
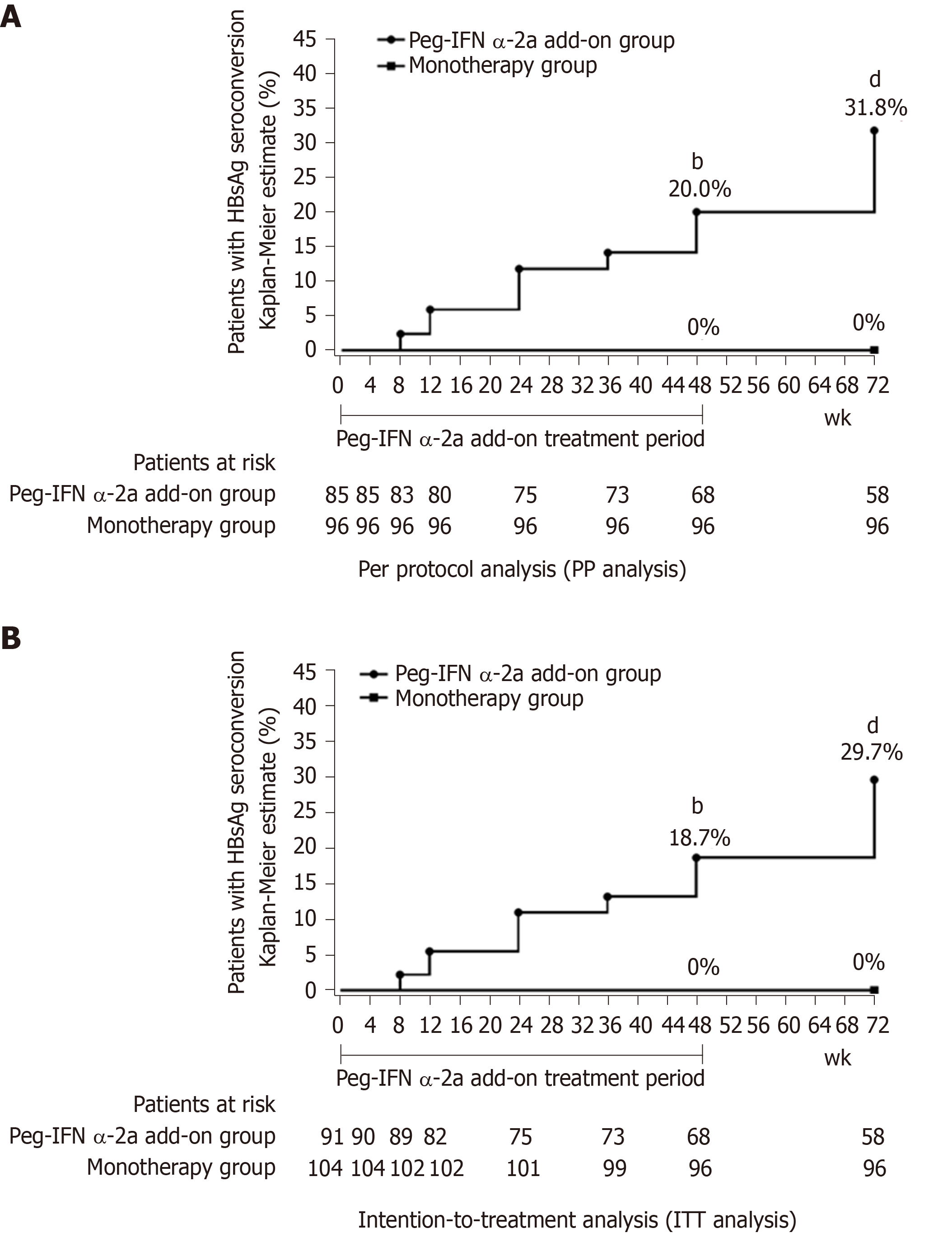
Figure 3 Hepatitis B surface antigen seroconversion rate.
A: Per protocol analysis showed that the rate of hepatitis B surface antigen seroconversion in 48-wk peg-IFN add-on group was significantly higher than monotherapy group at weeks 48 and 72 (bP < 0.001 vs monotherapy group at week 48; dP < 0.001 vs monotherapy group at week 72). Week 0 was defined as the time when the patients were enrolled in this study for patients in the monotherapy group; B: Intention-to-treatment analysis showed that the rate of hepatitis B surface antigen seroconversion in 48-wk peg-IFN add-on group was significantly higher than rate in monotherapy group at weeks 48 and 72 (bP < 0.001 vs monotherapy group at week 48; dP < 0.001 vs monotherapy group at week 72). Week 0 was defined as the time when the patients were enrolled in this study for patients in the monotherapy group. HBsAg: Hepatitis B surface antigen.
- Citation: Wu FP, Yang Y, Li M, Liu YX, Li YP, Wang WJ, Shi JJ, Zhang X, Jia XL, Dang SS. Add-on pegylated interferon augments hepatitis B surface antigen clearance vs continuous nucleos(t)ide analog monotherapy in Chinese patients with chronic hepatitis B and hepatitis B surface antigen ≤ 1500 IU/mL: An observational study. World J Gastroenterol 2020; 26(13): 1525-1539
- URL: https://www.wjgnet.com/1007-9327/full/v26/i13/1525.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i13.1525