Copyright
©The Author(s) 2019.
World J Gastroenterol. Oct 28, 2019; 25(40): 6158-6171
Published online Oct 28, 2019. doi: 10.3748/wjg.v25.i40.6158
Published online Oct 28, 2019. doi: 10.3748/wjg.v25.i40.6158
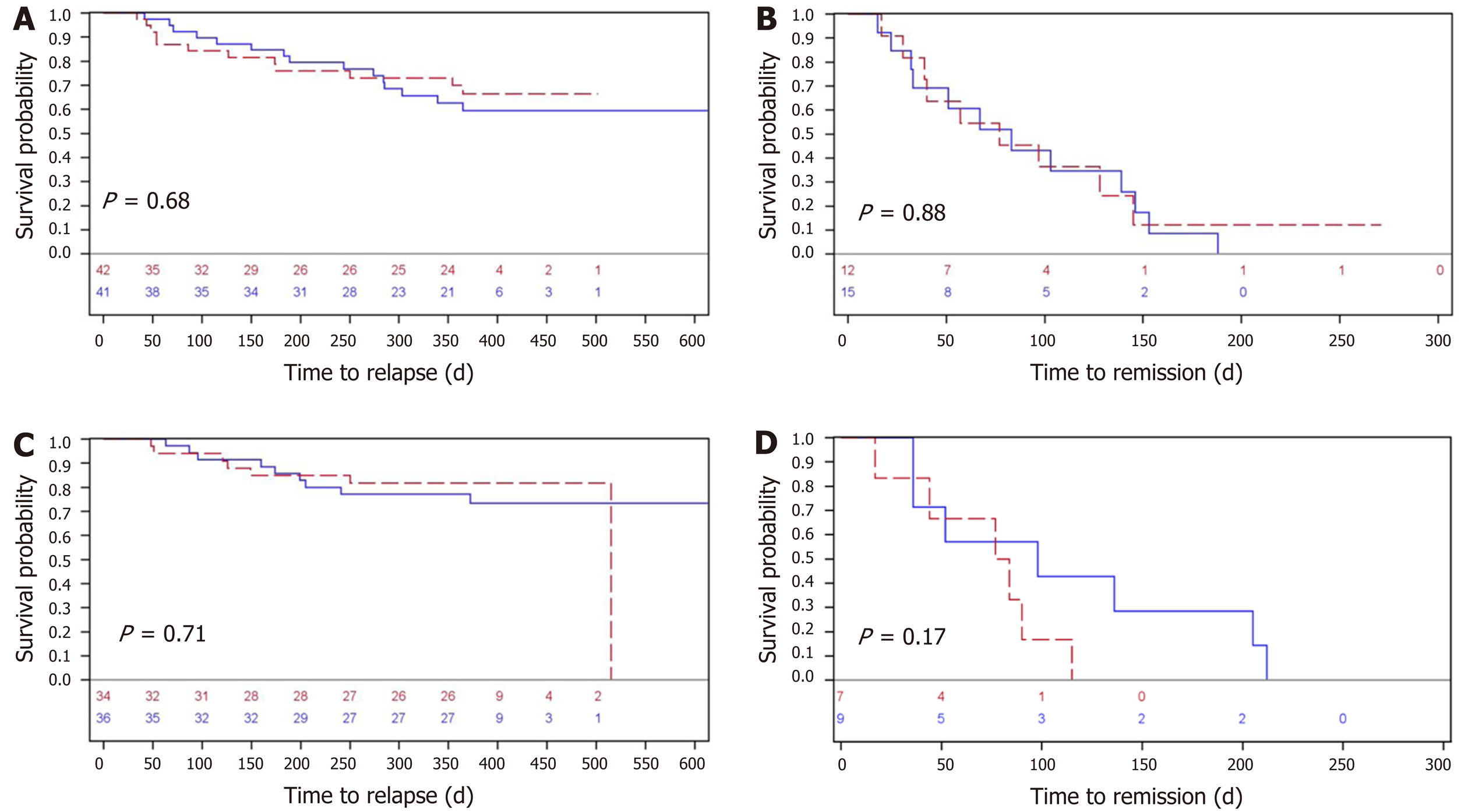
Figure 4 Analyzed by fecal calprotectin and simple clinical colitis activity index for all patients at risk.
A: Time to severe relapse (in days) measured by fecal calprotectin for all patients at risk (n = 83); B: The corresponding (days) to remission (n = 27); C: Time in days to a severe relapse measured by simple clinical colitis activity index for all patients with ulcerative colitis at risk (n = 70); D: The corresponding time (days) to remission (n = 16). The two electronic health screening procedures (every third month and on demand) are represented by blue and red lines, respectively.
- Citation: Ankersen DV, Weimers P, Marker D, Bennedsen M, Saboori S, Paridaens K, Burisch J, Munkholm P. Individualized home-monitoring of disease activity in adult patients with inflammatory bowel disease can be recommended in clinical practice: A randomized-clinical trial. World J Gastroenterol 2019; 25(40): 6158-6171
- URL: https://www.wjgnet.com/1007-9327/full/v25/i40/6158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i40.6158