Copyright
©The Author(s) 2019.
World J Gastroenterol. Oct 21, 2019; 25(39): 5961-5972
Published online Oct 21, 2019. doi: 10.3748/wjg.v25.i39.5961
Published online Oct 21, 2019. doi: 10.3748/wjg.v25.i39.5961
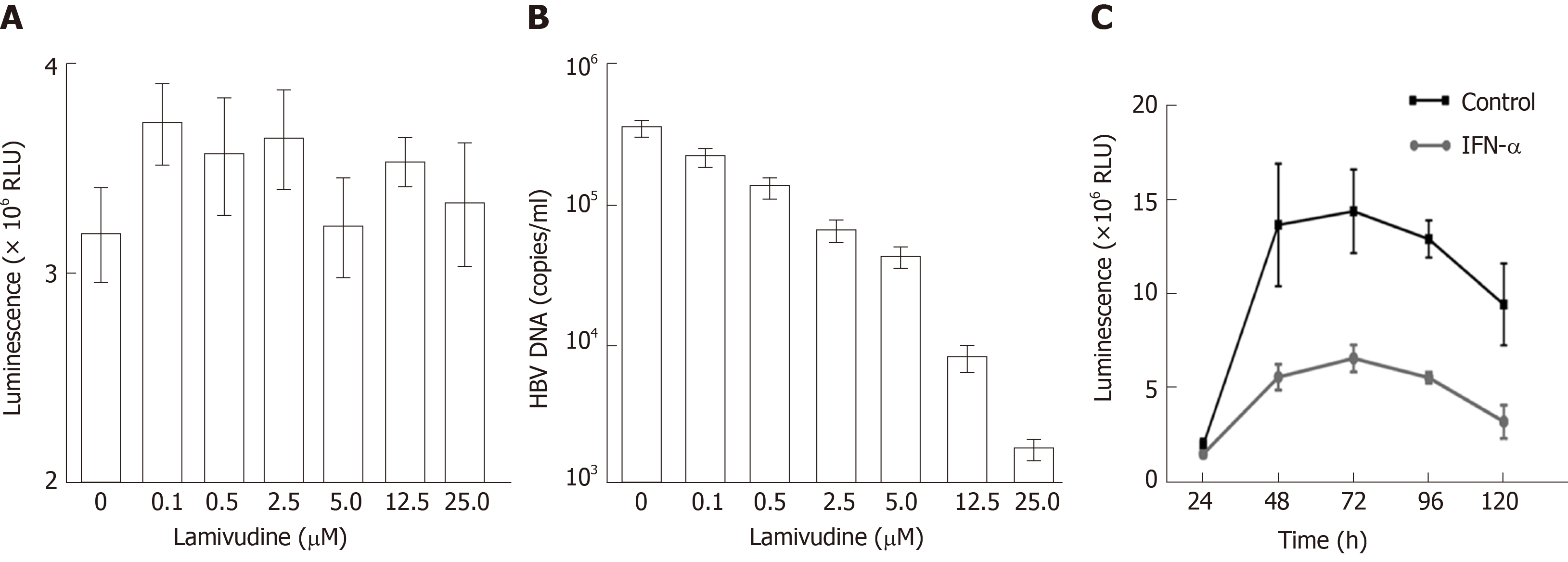
Figure 4 Drug susceptibility testing for the new hepatitis B virus -NLuc-35 cell lines.
A: Effect of lamivudine on luciferase expression in hepatitis B virus (HBV)-NLuc-35 cell lines. HBV-NLuc-35 cell lines were treated with increasing concentrations of lamivudine (0, 0.1, 0.5, 2.5, 5.0, 12.5, and 25.0 µM). After 72 h, luciferase expression in the supernatant was detected with Nano-Glo luciferase assay reagent; B: Cell line-borne HBV affected by lamivudine. The number of copies of HBV DNA in the supernatant was measured by qPCR; C: Effect of IFN-α on HBV-NLuc-35 cell lines. HBV-NLuc-35 cell lines were inoculated with 3000 IU/mL IFN-α (down line) or untreated (up line), and luciferase expression in the supernatant was detected at the indicated time points (24, 48, 72, 96, and 120 h). HBV: Hepatitis B virus; RUL: Relative light unit.
- Citation: Ruan J, Ping CY, Sun S, Cheng X, Han PY, Zhang YG, Sun DX. Construction of a replication-competent hepatitis B virus vector carrying secreted luciferase transgene and establishment of new hepatitis B virus replication and expression cell lines. World J Gastroenterol 2019; 25(39): 5961-5972
- URL: https://www.wjgnet.com/1007-9327/full/v25/i39/5961.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i39.5961