Copyright
©The Author(s) 2019.
World J Gastroenterol. Jul 7, 2019; 25(25): 3207-3217
Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3207
Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3207
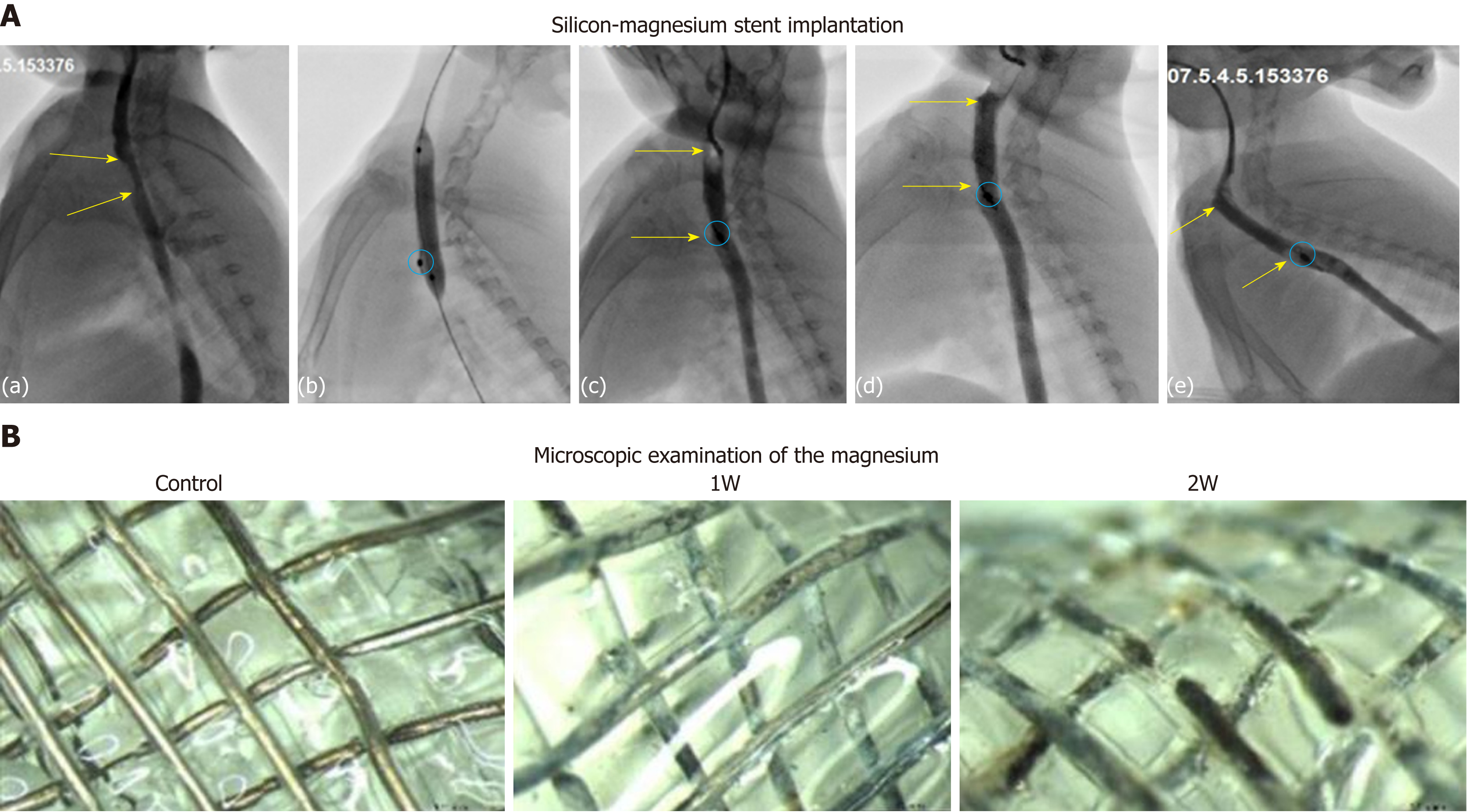
Figure 5 Implantation, follow-up, and in vivo degradation of silicone-coated magnesium stents.
A: Representative esophagography images show the procedure of stent insertion in rabbits. (a) Upper-middle esophageal stenosis (yellow arrow); (b) Balloon expansion before stent implantation; (c) Esophagography after stent implantation. Stenotic esophagus expansion and in place (yellow arrow) are shown, and positioning mark in the bottom of stent is clearly visible (red oval); (d-e) Follow-up at 1 wk (d) and 2 wk (e) after stent insertion. Stenotic esophagus expansion and in place (yellow arrow) are shown, with positioning mark clearly visible (red oval). B: Microscopic examination of the magnesium to track its retention before (control) and at 1 wk (1W) and 2 wk (2W) after stent implantation.
- Citation: Yang K, Cao J, Yuan TW, Zhu YQ, Zhou B, Cheng YS. Silicone-covered biodegradable magnesium stent for treating benign esophageal stricture in a rabbit model. World J Gastroenterol 2019; 25(25): 3207-3217
- URL: https://www.wjgnet.com/1007-9327/full/v25/i25/3207.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i25.3207