Copyright
©The Author(s) 2019.
World J Gastroenterol. Jul 7, 2019; 25(25): 3207-3217
Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3207
Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3207
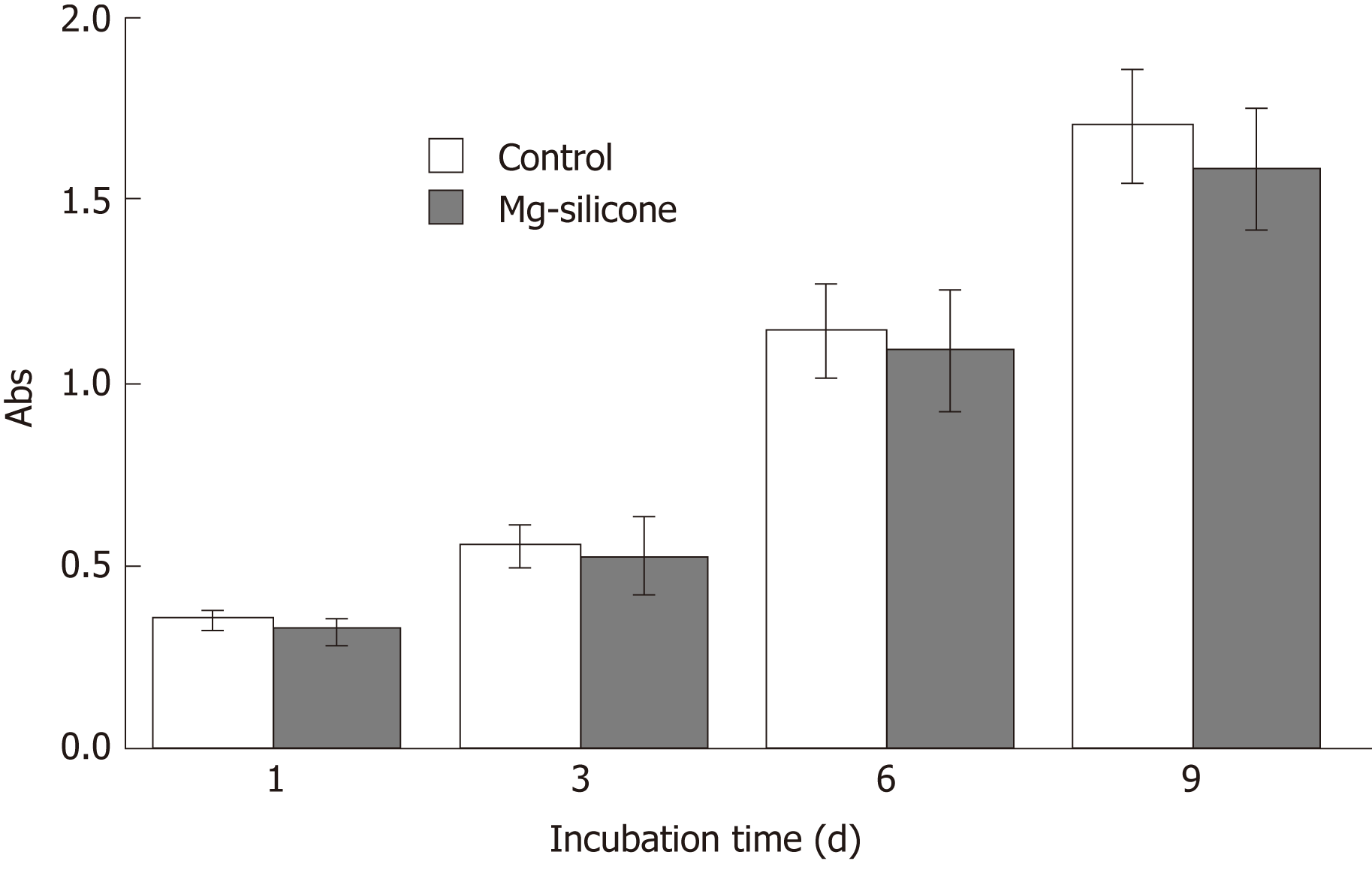
Figure 4 Biological safety evaluation of the magnesium-silicone material by testing the impact on proliferation of human smooth muscle cells.
Smooth muscle cells were cultured in the presence and absence of silicone-magnesium gel, and cell proliferation was assayed at 1, 3, 6, and 9 d after culturing (n = 3 for each group at each time point). Control, cultured without silicon-magnesium gel; Mg-silicone, cultured with silicone-magnesium gel.
- Citation: Yang K, Cao J, Yuan TW, Zhu YQ, Zhou B, Cheng YS. Silicone-covered biodegradable magnesium stent for treating benign esophageal stricture in a rabbit model. World J Gastroenterol 2019; 25(25): 3207-3217
- URL: https://www.wjgnet.com/1007-9327/full/v25/i25/3207.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i25.3207