Published online Jun 28, 2019. doi: 10.3748/wjg.v25.i24.3056
Peer-review started: February 22, 2019
First decision: February 26, 2019
Revised: May 28, 2019
Accepted: May 31, 2019
Article in press: June 1, 2019
Published online: June 28, 2019
Non-alcoholic fatty liver disease (NAFLD) is a common chronic liver disease worldwide. However, to date, there is no ideal therapy for this disease.
To study the effects of Si-Ni-San freeze-dried powder on high fat diet-induced NAFLD in mice.
Twenty-four male C57BL/6 mice were randomized into three groups of eight. The control group (CON) was allowed ad libitum access to a normal chow diet. The high fat diet group (FAT) and Si-Ni-San group (SNS) were allowed ad libitum access to a high fat diet. The SNS group was intragastrically administered Si-Ni-San freeze-dried powder (5.0 g/kg) once daily, and the CON and FAT groups were intragastrically administered distilled water. After 12 wk, body weight, liver index, visceral fat index, serum alanine aminotransferase (ALT), portal lipopoly-saccharide (LPS), liver tumor necrosis factor (TNF)-α and liver triglycerides were measured. Intestinal microbiota were analyzed using a 16S r DNA sequencing technique.
Compared with the FAT group, the SNS group exhibited decreased body weight, liver index, visceral fat index, serum ALT, portal LPS, liver TNF-α and liver triglycerides (P < 0.05). Intestinal microbiota analysis showed that the SNS group had different bacterial composition and function compared with the FAT group. In particular, Oscillospira genus was a bacterial biomarker of SNS group samples.
The beneficial effects of Si-Ni-San freeze-dried powder on high fat diet-induced NAFLD in mice may be associated with its anti-inflammatory and changing intestinal microbiota effects.
Core tip: We studied the effects of Si-Ni-San freeze-dried powder on high fat diet-induced nonalcoholic fatty liver disease (NAFLD) in mice. We found that Si-Ni-San freeze-dried powder ameliorated high fat diet-induced NAFLD in mice, and the mechanism of action of Si-Ni-San freeze-dried powder against NAFLD may be associated with its anti-inflammatory and changing intestinal microbiota effects. Our findings provide some useful information for therapy of NAFLD.
- Citation: Zhu F, Li YM, Feng TT, Wu Y, Zhang HX, Jin GY, Liu JP. Freeze-dried Si-Ni-San powder can ameliorate high fat diet-induced non-alcoholic fatty liver disease. World J Gastroenterol 2019; 25(24): 3056-3068
- URL: https://www.wjgnet.com/1007-9327/full/v25/i24/3056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i24.3056
Nonalcoholic fatty liver disease (NAFLD) is a condition of excess fat accumulation in the liver without significant alcohol consumption, and it consists of liver damage, ranging from steatosis to steatohepatitis, advanced fibrosis and cirrhosis. Obesity, hypertriglyceridemia, hyperglycemia, and type 2 diabetes are the best-known risk factors for NAFLD[1,2]. Besides these risk factors, plus-size clothing and sleep shortage may also be associated with NAFLD[3].
Over recent decades, we have witnessed a markedly increasing incidence of NAFLD, and it has become one of the most common chronic liver diseases worldwide[4,5]. In the United States, the proportion of patients with this chronic liver diseases rose from 47%-75%[6]. With the prevalent use of hepatitis vaccine and unchanged lifestyle, we can foresee that this proportion will continue to rise in the future. Nonalcoholic steatohepatitis (NASH), a more severe form of NAFLD, can lead to cirrhosis, liver failure, hepatocellular carcinoma and liver-related death[7-9]. In addition, NAFLD is a risk factor for cardiovascular diseases, diabetes and chronic kidney disease, and it also causes a high level of non-liver-related mortality[10,11]. Although NAFLD poses a threat to human health, the exact pathogenesis of this disease remains unclear. Moreover, until now, there are no approved medications for NAFLD treatment. Despite guidelines[12,13] recommending lifestyle modification as the first line treatment for NAFLD, compliance is a challenge. Therefore, it is important to look for new drug therapies.
Si-Ni-San, first recorded by Zhong-Jing Zhang during the Eastern Han Dynasty, is a famous prescription of traditional Chinese medicine to coordinate the functions of liver and spleen, and it has been used in China for thousands of years. This prescription consists of four herbal medicines: Bupleuri Radix, Paeoniae Alba Radix, Aurantii Immaturus Fructus, and Honey-fried Licorice Root in equal proportions. Si-Ni-San can alleviate liver injury through protecting hepatocyte membranes, increasing NO release and facilitating apoptosis of liver-infiltrating cells[14], and modified Si-Ni-San also has hepatoprotective effects[15]. Moreover, glycyrrhizin[16] and paeoniflorin[17], two components of Si-Ni-San, have shown beneficial effects on NAFLD. Therefore, we hypothesize that Si-Ni-San may have some beneficial effects against NAFLD.
The human intestine harbors 10-100 trillion microorganisms, mainly bacteria, collectively referred to as the intestinal microbiota[18,19]. The intestinal microbiota carries 150-fold more genes than the human genome, and these vast number of genes endow our body with special functions that we have not acquired during evolution, such as digesting plant polysaccharides[20,21]. Although the homeostasis of the intestinal microbiota is important for human health, dysbiosis of intestinal microbiota may cause disease. Previous studies have demonstrated that intestinal microbiota play a crucial role in the development of NAFLD[22-24], and patients with NAFLD have a different intestinal microbiota composition compared with healthy controls[25]. After oral administration, herbal medicines are exposed to the intestinal microbiota, and interactions are inevitable. Emerging studies have found that some herbal medicines can change the composition of the intestinal microbiota, which is viewed as an underlying therapeutic mechanism of herbal medicines[26,27]. In contrast, for other herbal medicines, such as berberine[28] and hesperidin[29], the intestinal microbiota play a critical role in mediating their therapeutic effects.
The purpose of the present study was to preliminarily investigate the therapeutic effect of Si-Ni-San freeze-dried powder on high fat diet-induced NAFLD in a mouse model, and its effect on the composition and function of the intestinal microbiota, which will provide us with a deeper understanding of the therapeutic mechanism of Si-Ni-San freeze-dried powder.
Bupleuri Radix, Paeoniae Alba Radix, Aurantii Immaturus Fructus, and Honey-fried Licorice Root were purchased from Beijing Tongrentang (Beijing, China) and were authenticated by our team. The herbs were mixed at a mass ratio of 1:1:1:1, and the mixture (2000 g) was decocted with distilled water and then filtered. The filtrate was prepared by freeze-drying, and five major constituents in the freeze-dried powder were quantified by HPLC (Table 1).
Twenty-four male 5-wk-old C57BL/6 mice (Beijing Vital River Laboratory Animal Technology Co. Ltd., Beijing, China) were acclimated for 1 wk at a temperature of 20-22 °C and humidity of 40%-45% in controlled rooms with an alternating 12-h light and dark cycle. After acclimation, mice were randomized into three groups of eight. The control group (CON) was allowed ad libitum access to a normal chow diet for 12 wk. The high fat diet group (FAT) and Si-Ni-San group (SNS) were allowed ad libitum access to a high fat diet for 12 wk. The composition of normal chow diet and high fat diet are shown in Table 2. SNS group mice were intragastrically administered Si-Ni-San freeze-dried powder (5.0 g/kg) once daily. The CON and FAT groups were intragastrically administered distilled water once daily. The study was approved by the Animal Ethics Committee of Hebei North University (No. 2016-1-0-06).
| Normal chow diet in g/kg | High fat diet in g/kg | |
| Casein | 212.33 | 261.02 |
| L-Cystine | 2.84 | 3.50 |
| Corn starch | 275.84 | 56.87 |
| Maltodextrin | 33.18 | 116.53 |
| Sucrose | 331.77 | 201.36 |
| Cellulose | 47.40 | 58.26 |
| Soybean oil | 23.70 | 29.13 |
| Lard | 18.96 | 206.84 |
| Mineral mix | 9.84 | 11.65 |
| Dicalcium phosphate | 12.32 | 15.15 |
| Calcium carbonate | 5.21 | 6.41 |
| Potassium citrate | 15.64 | 19.23 |
| Vitamin mix | 9.48 | 11.56 |
| Choline bitartrate | 1.90 | 2.33 |
| FD&C dye | 0.047 | 0.058 |
After 12 wk treatment, all mice were anesthetized with ketamine (80 mg/kg) and xylazine (6 mg/kg). The liver, mesenteric fat, retroperitoneal fat and epididymal fat were isolated and weighed. Liver index was calculated as the ratio of liver to body weight. The visceral fat index was calculated as the ratio of visceral fat (mesenteric, retroperitoneal and epididymal fat) to body weight.
Liver lipid was extracted as previously described[30], and liver triglycerides were measured using a Triglyceride Reagent Kit (Dongou, Wenzhou, China). Frozen sections stained with Oil Red O were also used for hepatic lipid detection.
When the mice were killed, blood samples were collected via cardiac puncture and centrifuged. Serum alanine aminotransferase (ALT) was measured by standard procedures. One milliliter of portal blood was collected for analysis by lipopolysaccharide (LPS) assay. The level of portal LPS was measured using a chromogenic limulus amoebocyte lysate test kit (Bokang, Zhanjiang, China) according to the manufacturer’s instructions.
Portions of liver tissues were homogenized (100 mg/mL) in RIPA lysis buffer (Beyotime, Shanghai, China). Liver TNF-α was measured using Mouse TNF-alpha Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, United States).
One week before the mice were killed, fecal samples were collected and stored at -80 °C. The total fecal DNA was extracted using QIAamp Fast DNA Stool Mini Kit (QIAGEN, Valencia, CA, United States). V4 hypervariable region of 16S rRNA genes was amplified using specific primers (515F: 5'-GTGCCAGCMGCCGCGGTAA-3', 806R: 5'-GGACTACHVGGGTWTCTAAT-3'). PCR products were mixed in equidensity ratios. Sequencing libraries were prepared using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, United States). The library was sequenced on an Illumina HiSeq2500 platform and paired-end reads were generated.
QIIME pipeline (1.9.1) was used to process and analyze the raw data[31]. The main scripts used in our study were as follows: join_paired_ends.py, split_lib-raries_fastq.py, pick_open_reference_otus.py and core_diversity_analyses.py. Operational taxonomic units (OTUs) were clustered at 97% similarity, and sequences were taxonomically assigned against the Greengenes database (gg_13_8). The results of α diversity, β diversity and bacterial taxonomy were generated by core_diver-sity_analyses.py script. We used the linear discriminant analysis (LDA) effect size (LEfSe) method to identify bacterial biomarkers in different groups[32]. We predicted the bacterial functions of different groups. For functional prediction, we reclustered sequences into OTUs (97% similarity) against the Greengenes database (gg_13_5) using pick_closed_reference_otus.py script, and then the PICRUSt pipeline[33] was used to predict bacterial functions. After using the norma-lize_by_copy_number.py script, the predict_metagenomes.py script was used to generate Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog predictions. We also applied the LEfSe method to find functional biomarkers in different groups.
Data are presented as mean ± SD. The differences in data were statistically analyzed by one-way analysis of variance with Bonferroni’s multiple-comparison test as post hoc analysis. SPSS version 20.0 was used for statistical analysis, and P < 0.05 was considered statistically significant.
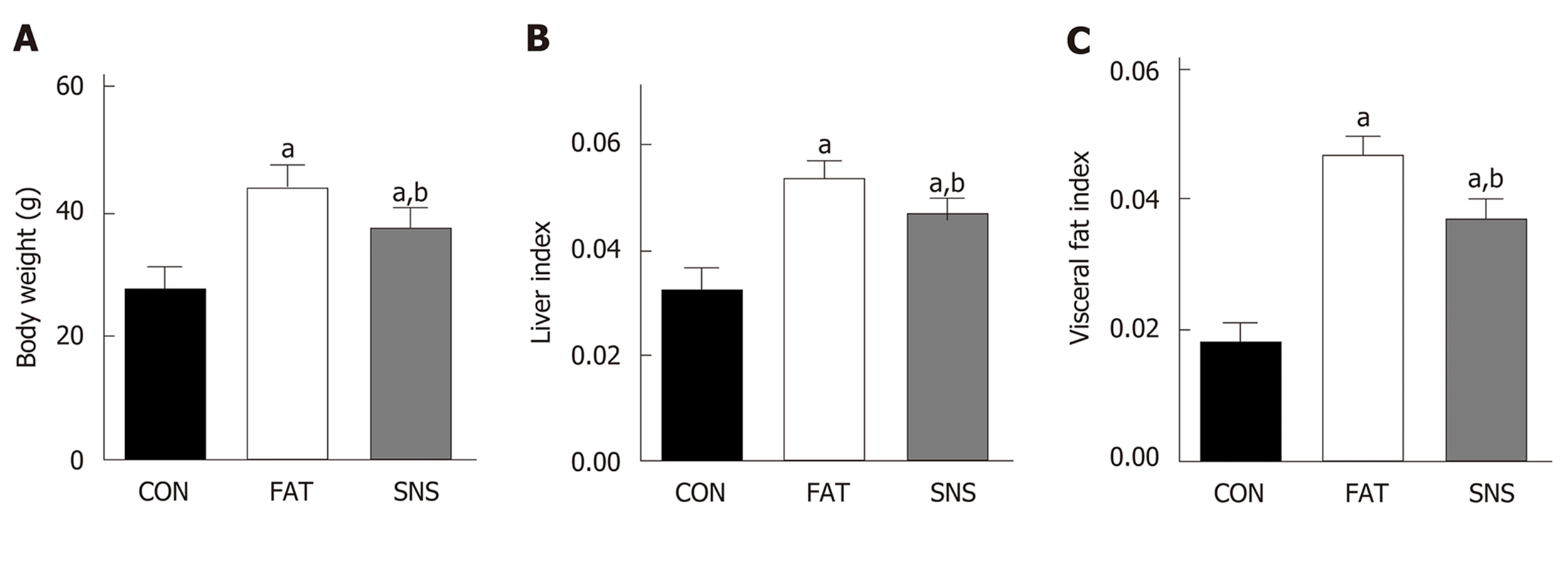
At the beginning of the experiment, the body weight of the three groups of mice did not differ significantly (data not shown). After 12 wk, the FAT and SNS groups exhibited higher body weight, liver index and visceral fat index compared with the CON group (Figure 1A-C, P < 0.05, respectively). However, the SNS group, compared with the FAT group, showed decreased body weight, liver index and visceral fat index (Figure 1A-C, P < 0.05, respectively).
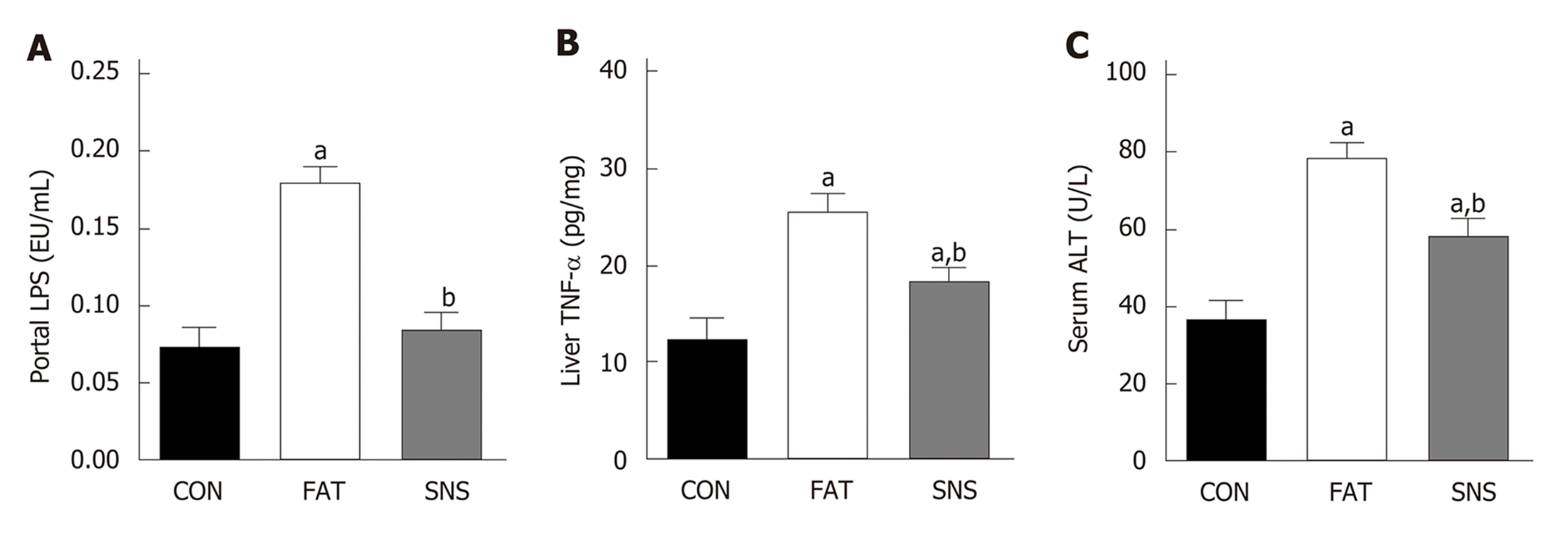
After 12 wk of eating the high fat diet, the FAT group had significantly increased levels of portal LPS, liver TNF-α and ALT compared with the CON group (Figure 2A-C, P < 0.05, respectively). However, compared with the FAT group, the SNS group, which was intragastrically administered Si-Ni-San freeze-dried powder every day for 12 wk, significantly decreased levels of portal LPS, liver TNF-α and ALT (Figure 2A-C, P < 0.05, respectively). Compared with the CON group, the SNS group only exhibited higher levels of liver TNF-α and ALT (Figure 2B and C, P < 0.05, respectively).
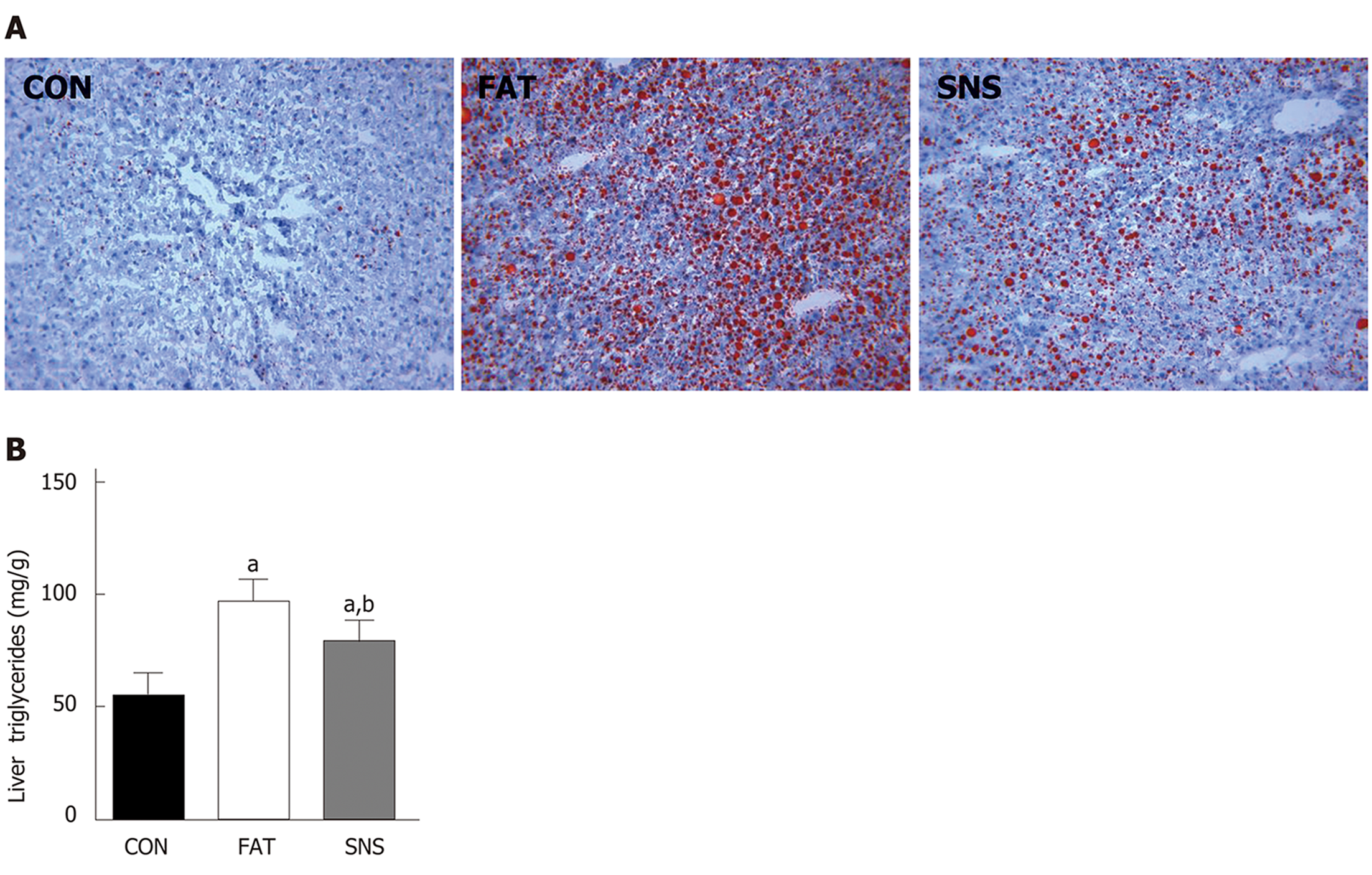
Oil Red O staining was used to morphologically observe triglyceride accumulation in the livers of mice. The CON group nearly had no triglyceride accumulation in the liver; however, the FAT group had obvious triglyceride accumulation (Figure 3A). The SNS group exhibited less triglyceride accumulation compared with the FAT group (Figure 3A). Triglyceride accumulation was measured using a colorimetric method, and the results were consistent with the findings of Oil Red O staining. The levels of triglycerides were significantly increased in the liver of the FAT and SNS groups compared with the CON group (Figure 3B, P < 0.05). Si-Ni-San freeze-dried powder significantly decreased the levels of triglycerides in the SNS group compared with the FAT group (Figure 3B, P < 0.05).
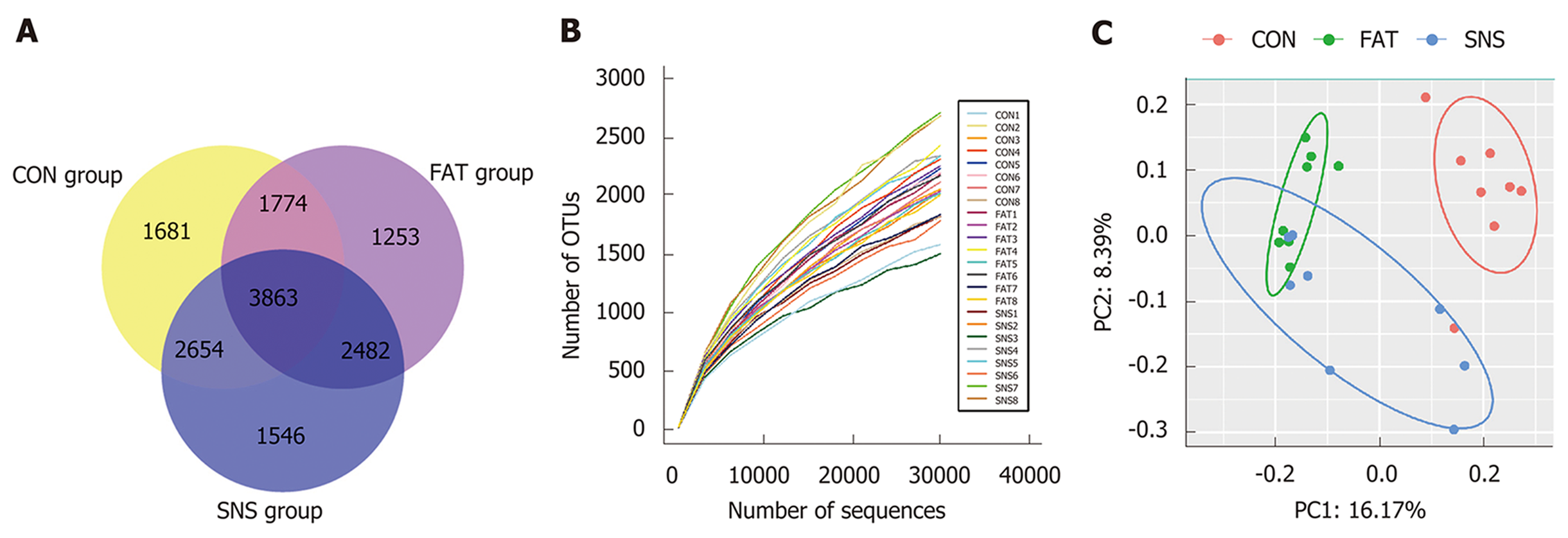
A total of 1,380,651 high-quality reads (average of 57,527 ± 14,266 sequences per sample) were used for biological information analysis, and 15,253 OTUs were clustered at 97% similarity (Figure 4A). Good coverage, which was > 0.96, and the rarefaction curves of all samples indicated that we obtained sufficient OTUs to reflect the bacterial composition of different samples accurately (Figure 4B). Although we calculated the values of Shannon Index and Chao 1, the three groups of mice showed no significant differences (date not shown). To observe the β diversity of different groups of mice, we used Unweighted UniFrac PCoA, and the result of this evolutionary distance-based method showed that the intestinal microbiota of the CON group was obviously separated from that of the FAT and SNS groups (Figure 4C). Despite partially overlapping, the intestinal microbiota of the FAT and SNS groups were roughly separated (Figure 4C). Analysis of molecular variance was used to further assess the spatial separation, and revealed that samples from the three groups differed significantly (CON vs FAT group, P < 0.001; FAT vs SNS group, P < 0.001; CON vs SNS group, P < 0.001).
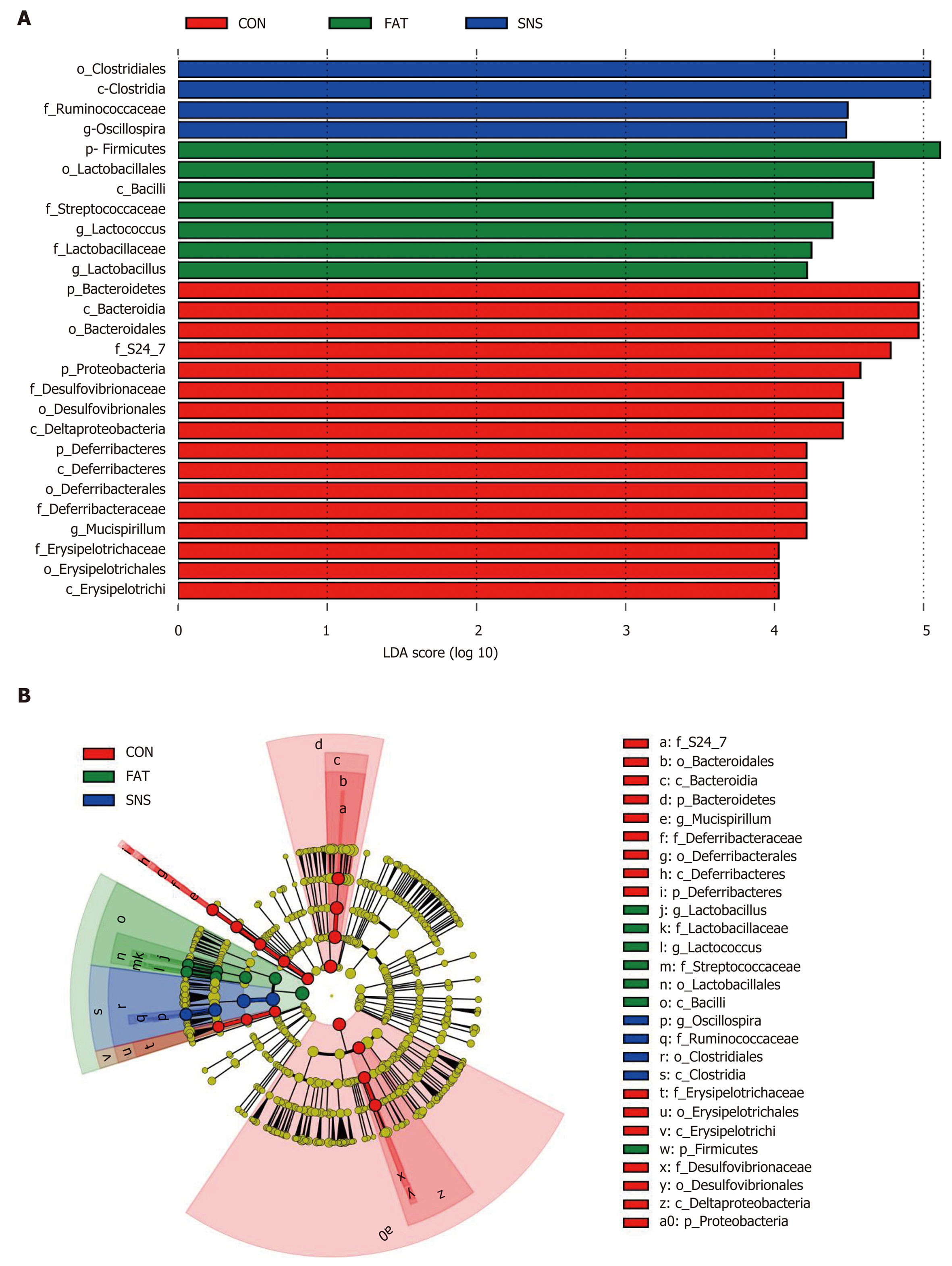
At the phylum level, 27 bacterial phyla were identified, and the top five were Firmicutes, Bacteroidetes, Proteobacteria, Deferribacteres and Actinobacteria. The phyla of BRC1, Chlamydiae, Chlorobi, Fibrobacteres, GN04 and OP3 were only found in the CON and SNS groups. Moreover, the bacterial phyla of Synergistetes and TM6 were only found in the SNS group. The FAT group samples increased the ratio of Firmicutes to Bacteroidetes compared with the CON group samples; however, this ratio in the SNS group samples did not differ significantly compared with the CON and FAT group samples. At the genus level, 227 genera were identified. To find taxon differences, we used the LEfSe method and showed that S24-7 (family level), Bacteroidales (order level), Bacteroidia (class level), Bacteroidetes (phylum level), Mucispirillum (genus level), Deferribacteraceae (family level), Deferribacterales (order level), Deferribacteres (class level), Deferribacteres (phylum level), Erysipelotri-chaceae (family level), Erysipelotrichales (order level), Erysipelotrichi (class level), Desulfovibrionaceae (family level), Desulfovibrionales (order level), Delta-proteobacteria (class level), and Proteobacteria (phylum level) were biomarkers in the CON group samples (Figure 5A and B). Lactobacillus (genus level), Lactobacillaceae (family level), Lactococcus (genus level), Streptococcaceae (family level), Lactobacillales (order level), Bacilli (class level), and Firmicutes (phylum level) were biomarkers in the FAT group samples (Figure 5A and B). Oscillospira (genus level), Ruminococcaceae (family level), Clostridiales (order level), and Clostridia (class level) were biomarkers in the SNS group samples (Figure 5A and B).
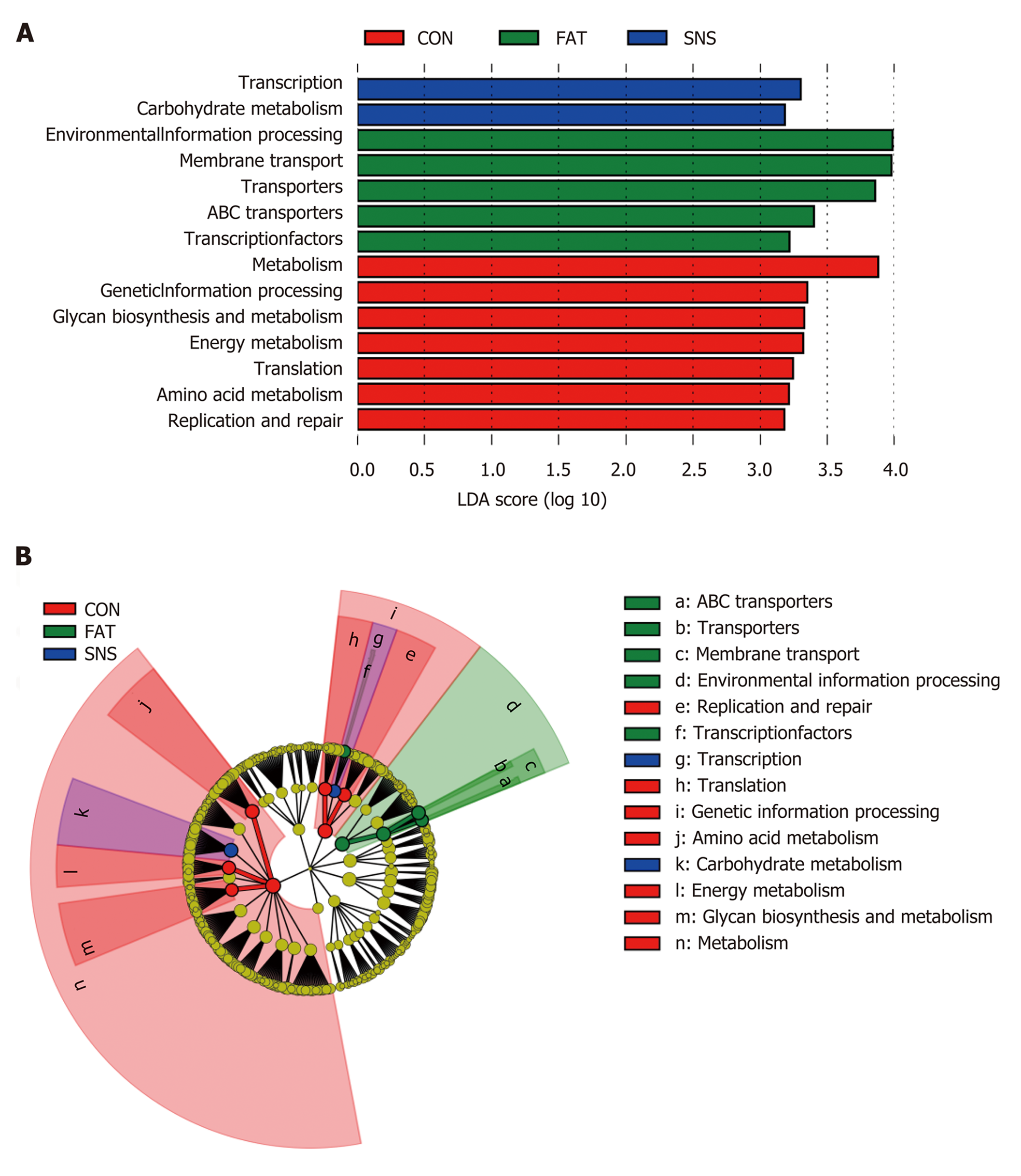
We used PICRUSt to predict the functions of bacterial microbiota using KEGG database at level 3, and then the LEfSe method to find functional biomarkers in different group samples. LEfSe showed that metabolism, genetic information processing, glycan biosynthesis and metabolism, energy metabolism, translation, amino acid metabolism, and replication and repair were functional biomarkers of the CON group samples (Figure 6A and B). Transcription and carbohydrate metabolism were functional biomarkers of the SNS group samples (Figure 6A and B). Environmental information processing, transporters, ABC transporters and transcription factors were functional biomarkers of the FAT group samples (Figure 6A and B).
The present study preliminarily investigated the effects of Si-Ni-San freeze-dried powder on high fat diet-induced NAFLD in mice. We demonstrated that Si-Ni-San freeze-dried powder ameliorates high fat diet-induced high levels of body weight, liver index, visceral fat index, portal LPS, serum ALT, liver TNF-α and liver triglyceride accumulation in mice. We also found that Si-Ni-San freeze-dried powder can alter intestinal microbiota composition and function in mice.
Previous research has studied the effects of Si-Ni-San on NAFLD, and found that Si-Ni-San can reduce the liver concentration of total cholesterol, triglyceride, free fatty acid and interleukin (IL)-6 in rats with NAFLD[34]. However, animal models of NAFLD, in this research, were induced using a long-term chronic stress method that is not widely used in NAFLD studies. In addition, this method caused a decrease in body weight compared with control rats, which does not match the real-life situation. In the present study, we adopted a high fat diet to induce animal models of NAFLD, which is a stable and widely used method in NAFLD studies. This method always induces obesity, insulin resistance, dyslipidemia and liver triglyceride accumulation, which more accurately reflect real-life situations. Thus, our present study has a wider practical significance.
NAFLD is always considered to be the hepatic manifestation of metabolic syndrome, and insulin resistance plays an important role in the pathogenesis of NAFLD[35-37]. In the present study, although the level of insulin resistance in the SNS group exhibited a decreased trend compared with the FAT group, the difference was not significant (data not shown), suggesting that improving insulin resistance is not a mechanism of action of Si-Ni-San freeze-dried powder, or its improvement of insulin resistance is not due to small sample size. Thus, additional large-sample studies addressing the effect of Si-Ni-San freeze-dried powder on insulin resistance are warranted.
Previous studies have revealed the key role of LPS, a constituent of Gram-negative bacteria and main constituent of endotoxemia, in the development of metabolic diseases, and elevated LPS has been observed in patients with NAFLD[25,38]. LPS is an inflammatory trigger, and it can promote the secretion of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1 and IL-6. In the present study, we found that high fat diet increased the levels of portal LPS and liver TNF-α compared with normal chow diet; however, Si-Ni-San freeze-dried powder reduced these increases, suggesting that the anti-inflammatory effect of Si-Ni-San freeze-dried powder may be a mechanism underlying its beneficial effect. Although the portal LPS of the CON and SNS groups did not differ significantly, the SNS group only exhibited higher levels of liver TNF-α and ALT, suggesting that in addition to LPS, there may be other proinflammatory factors.
The important role of intestinal microbiota in the pathogenesis of NAFLD has been revealed in previous studies[22,39]. Given the tight links between intestinal microbiota and NAFLD, manipulation of the intestinal microbiota of NAFLD is a promising therapeutic strategy[40-42]. Thus, in the present study, we investigated the effect of Si-Ni-San freeze-dried powder on intestinal microbiota, and found that the intestinal microbiota composition and function in the SNS group differed clearly from those in the FAT group, suggesting that Si-Ni-San freeze-dried powder alters high fat diet-induced intestinal microbiota dysbiosis. So, the therapeutic effect of Si-Ni-San freeze-dried powder on high fat diet-induced NAFLD may be achieved by changing the intestinal microbiota. The fact that the Oscillospira genus was a bacterial biomarker in the SNS group samples demands our further attention. Up to now, there has been little knowledge about this genus, mainly because it has never been cultured. Our former study found that Oscillospira is associated with a high protein diet, which has beneficial effects on NAFLD[30]. Other studies have found that Oscillospira is significantly reduced in patients with NASH or inflammatory bowel disease[43-45], and is positively associated with leanness[46]. A recent study has found that some Oscillospira species may be able to utilize glycans to secrete butyrate[47], which can prevent inflammation[48], and plays an important role in metabolic diseases[49-51]. Using a combination of our results with those of former studies, we infer that Si-Ni-San freeze-dried powder may achieve its beneficial effect by providing more special glycans to Oscillospira bacteria and then producing more butyrate, which has multiple beneficial effects on host health. The result that carbohydrate metabolism was a functional biomarker in the SNS group partly supports our inference, and further studies are warranted to confirm our hypothesis.
An interesting finding in our study was that Lactobacillus genus was a bacterial biomarker of the FAT group. Some strains of Lactobacillus are frequently used as probiotics[52]. Thus, our finding needs confirmation by further studies.
There were several limitations to the present study. First, there was a lack of metabonomics analysis, so the effects of Si-Ni-San freeze-dried powder on the metabolism of intestinal microbiota are not known. Second, we did not administer Si-Ni-San freeze-dried powder to normal chow diet mice, so the effects of Si-Ni-San freeze-dried powder on normal chow diet mice are not known. Third, the sample size was small, so our results need further studies for confirmation. Fourth, the change in some cytokines, such as IL-1 and IL-6, was not detected in the present study. These limitations will be addressed in our future studies.
In conclusion, our present study preliminarily confirmed the beneficial effects of Si-Ni-San freeze-dried powder on high fat diet-induced NAFLD in mice, and that the mechanisms of action of Si-Ni-San freeze-dried powder against NAFLD may be associated with its anti-inflammatory effects and its changes to the intestinal microbiota. Our findings provide some useful information for NAFLD therapy. We provide the basis for the clinical use of Si-Ni-San freeze-dried powder and some underlying mechanisms of its action. Although more in-depth research is needed in the future, Si-Ni-San freeze-dried powder may also be a clinical option for NAFLD treatment.
The incidence of nonalcoholic fatty liver disease (NAFLD) dramatically increased in the last few decades. Unfortunately, until now, the clinical treatment of this common chronic liver disease is difficult, and some new effective therapies are needed.
Some herbal medicines have hepatoprotective effects, so we want to know if some famous prescriptions of traditional Chinese medicine can provide beneficial effects on NAFLD.
To explore the effects of Si-Ni-San, a famous prescription of traditional Chinese medicine, on NAFLD and intestinal microbiota.
We intragastrically administered Si-Ni-San freeze-dried powder (5.0 g/kg) to mice, which were allowed ad libitum access to a high fat diet. After 12 wk of treatment, we measured body weight, liver index, visceral fat index, serum alanine aminotransferase (ALT), portal lipopolysaccharide (LPS), liver tumor necrosis factor (TNF)-α, liver triglycerides and intestinal microbiota, and we compared the results of these parameters with mice in another group to find whether Si-Ni-San freeze-dried powder have some beneficial effects on NAFLD.
After Si-Ni-San freeze-dried powder treatment, the levels of body weight, liver index, visceral fat index, serum ALT, portal LPS, liver TNF-α and liver triglycerides were improved. The composition of intestinal microbiota was also changed, especially the Oscillospira genus.
Si-Ni-San freeze-dried powder can ameliorate NAFLD by an anti-inflammatory action and intestinal microbiota-changing effect.
Although we provide basis for the clinical use of Si-Ni-San freeze-dried powder and some underlying mechanisms of its action, the effects of Si-Ni-San freeze-dried powder on the metabolism of intestinal microbiota and some cytokines, such as interleukin (IL)-1 and IL-6, need to be addressed in future studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Musumeci G, Tarantino G S-Editor: Ma RY L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;16:1221-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3655] [Cited by in F6Publishing: 3620] [Article Influence: 164.5] [Reference Citation Analysis (2)] |
| 2. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [PubMed] [Cited in This Article: ] |
| 3. | Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G, Trovato GM. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Ray K. NAFLD-the next global epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1127] [Cited by in F6Publishing: 1224] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 6. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 759] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 7. | Gariani K, Philippe J, Jornayvaz FR. Non-alcoholic fatty liver disease and insulin resistance: from bench to bedside. Diabetes Metab. 2013;39:16-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1488] [Cited by in F6Publishing: 1434] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 9. | Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217-9228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 37] [Reference Citation Analysis (1)] |
| 10. | Kwak MS, Kim D. Long-term outcomes of nonalcoholic fatty liver disease. Curr Hepatol Rep. 2015;14:69-76. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3544] [Cited by in F6Publishing: 4139] [Article Influence: 689.8] [Reference Citation Analysis (8)] |
| 13. | European Association for the Study of the Liver; European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 405] [Article Influence: 50.6] [Reference Citation Analysis (2)] |
| 14. | Jiang J, Zhou C, Xu Q. Alleviating effects of si-ni-san, a traditional Chinese prescription, on experimental liver injury and its mechanisms. Biol Pharm Bull. 2003;26:1089-1094. [PubMed] [Cited in This Article: ] |
| 15. | Liu CG, Wang XL, Du XW, Jiang DY, Geng NZ, Zhang SX, Zhou YY, Kuang HX. Metabolomic profiling for identification of potential biomarkers in the protective effects of modified Sinisan against liver injury in dimethylnitrosamine treated rats. Biol Pharm Bull. 2013;36:1700-1707. [PubMed] [Cited in This Article: ] |
| 16. | Wu X, Zhang L, Gurley E, Studer E, Shang J, Wang T, Wang C, Yan M, Jiang Z, Hylemon PB, Sanyal AJ, Pandak WM, Zhou H. Prevention of free fatty acid-induced hepatic lipotoxicity by 18beta-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology. 2008;47:1905-1915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Zhang L, Yang B, Yu B. Paeoniflorin Protects against Nonalcoholic Fatty Liver Disease Induced by a High-Fat Diet in Mice. Biol Pharm Bull. 2015;38:1005-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2236] [Cited by in F6Publishing: 2335] [Article Influence: 194.6] [Reference Citation Analysis (0)] |
| 19. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7052] [Cited by in F6Publishing: 7170] [Article Influence: 512.1] [Reference Citation Analysis (3)] |
| 20. | Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578-6583. [PubMed] [Cited in This Article: ] |
| 21. | Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J; MetaHIT Consortium, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M'rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4310] [Cited by in F6Publishing: 4518] [Article Influence: 347.5] [Reference Citation Analysis (0)] |
| 22. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4530] [Cited by in F6Publishing: 4136] [Article Influence: 206.8] [Reference Citation Analysis (4)] |
| 23. | Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1778] [Cited by in F6Publishing: 1764] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 24. | Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948-4959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 382] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 25. | Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 503] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 26. | Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS One. 2011;6:e24520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Yin X, Peng J, Zhao L, Yu Y, Zhang X, Liu P, Feng Q, Hu Y, Pang X. Structural changes of gut microbiota in a rat non-alcoholic fatty liver disease model treated with a Chinese herbal formula. Syst Appl Microbiol. 2013;36:188-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Feng R, Shou JW, Zhao ZX, He CY, Ma C, Huang M, Fu J, Tan XS, Li XY, Wen BY, Chen X, Yang XY, Ren G, Lin Y, Chen Y, You XF, Wang Y, Jiang JD. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci Rep. 2015;5:12155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 29. | Amaretti A, Raimondi S, Leonardi A, Quartieri A, Rossi M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients. 2015;7:2788-2800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Liu JP, Zou WL, Chen SJ, Wei HY, Yin YN, Zou YY, Lu FG. Effects of different diets on intestinal microbiota and nonalcoholic fatty liver disease development. World J Gastroenterol. 2016;22:7353-7364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 50] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 31. | Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23810] [Cited by in F6Publishing: 22505] [Article Influence: 1607.5] [Reference Citation Analysis (0)] |
| 32. | Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7042] [Cited by in F6Publishing: 8785] [Article Influence: 675.8] [Reference Citation Analysis (0)] |
| 33. | Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5694] [Cited by in F6Publishing: 5809] [Article Influence: 528.1] [Reference Citation Analysis (0)] |
| 34. | Cheng F, Ma C, Wang X, Zhai C, Wang G, Xu X, Mu J, Li C, Wang Z, Zhang X, Yue W, Du X, Lian Y, Zhu W, Yin X, Wei Z, Song W, Wang Q. Effect of traditional Chinese medicine formula Sinisan on chronic restraint stress-induced nonalcoholic fatty liver disease: a rat study. BMC Complement Altern Med. 2017;17:203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [PubMed] [Cited in This Article: ] |
| 36. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [PubMed] [Cited in This Article: ] |
| 37. | Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:575-594, ix. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4095] [Cited by in F6Publishing: 4166] [Article Influence: 245.1] [Reference Citation Analysis (0)] |
| 39. | Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787-1794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 622] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 40. | Xue L, He J, Gao N, Lu X, Li M, Wu X, Liu Z, Jin Y, Liu J, Xu J, Geng Y. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. 2017;7:45176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 41. | Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, Liu C, Chen YW, Fan JG. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23:60-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 235] [Cited by in F6Publishing: 257] [Article Influence: 36.7] [Reference Citation Analysis (5)] |
| 42. | Cao Y, Pan Q, Cai W, Shen F, Chen GY, Xu LM, Fan JG. Modulation of Gut Microbiota by Berberine Improves Steatohepatitis in High-Fat Diet-Fed BALB/C Mice. Arch Iran Med. 2016;19:197-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 20] [Reference Citation Analysis (0)] |
| 43. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 1120] [Article Influence: 101.8] [Reference Citation Analysis (1)] |
| 44. | Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis C, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G, Dallapiccola B, Miccheli A, Alisi A, Putignani L. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65:451-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 464] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 45. | Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223-4233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 548] [Cited by in F6Publishing: 587] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 46. | Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW, Buurman WA, de Vos WM, Rensen SS. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring). 2013;21:E607-E615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 47. | Gophna U, Konikoff T, Nielsen HB. Oscillospira and related bacteria - From metagenomic species to metabolic features. Environ Microbiol. 2017;19:835-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 48. | Cushing K, Alvarado DM, Ciorba MA. Butyrate and Mucosal Inflammation: New Scientific Evidence Supports Clinical Observation. Clin Transl Gastroenterol. 2015;6:e108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3971] [Cited by in F6Publishing: 4344] [Article Influence: 362.0] [Reference Citation Analysis (0)] |
| 50. | Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1766] [Cited by in F6Publishing: 1823] [Article Influence: 165.7] [Reference Citation Analysis (0)] |
| 51. | Arora T, Bäckhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med. 2016;280:339-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 52. | Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 606] [Cited by in F6Publishing: 598] [Article Influence: 49.8] [Reference Citation Analysis (0)] |