Copyright
©The Author(s) 2019.
World J Gastroenterol. May 7, 2019; 25(17): 2110-2121
Published online May 7, 2019. doi: 10.3748/wjg.v25.i17.2110
Published online May 7, 2019. doi: 10.3748/wjg.v25.i17.2110
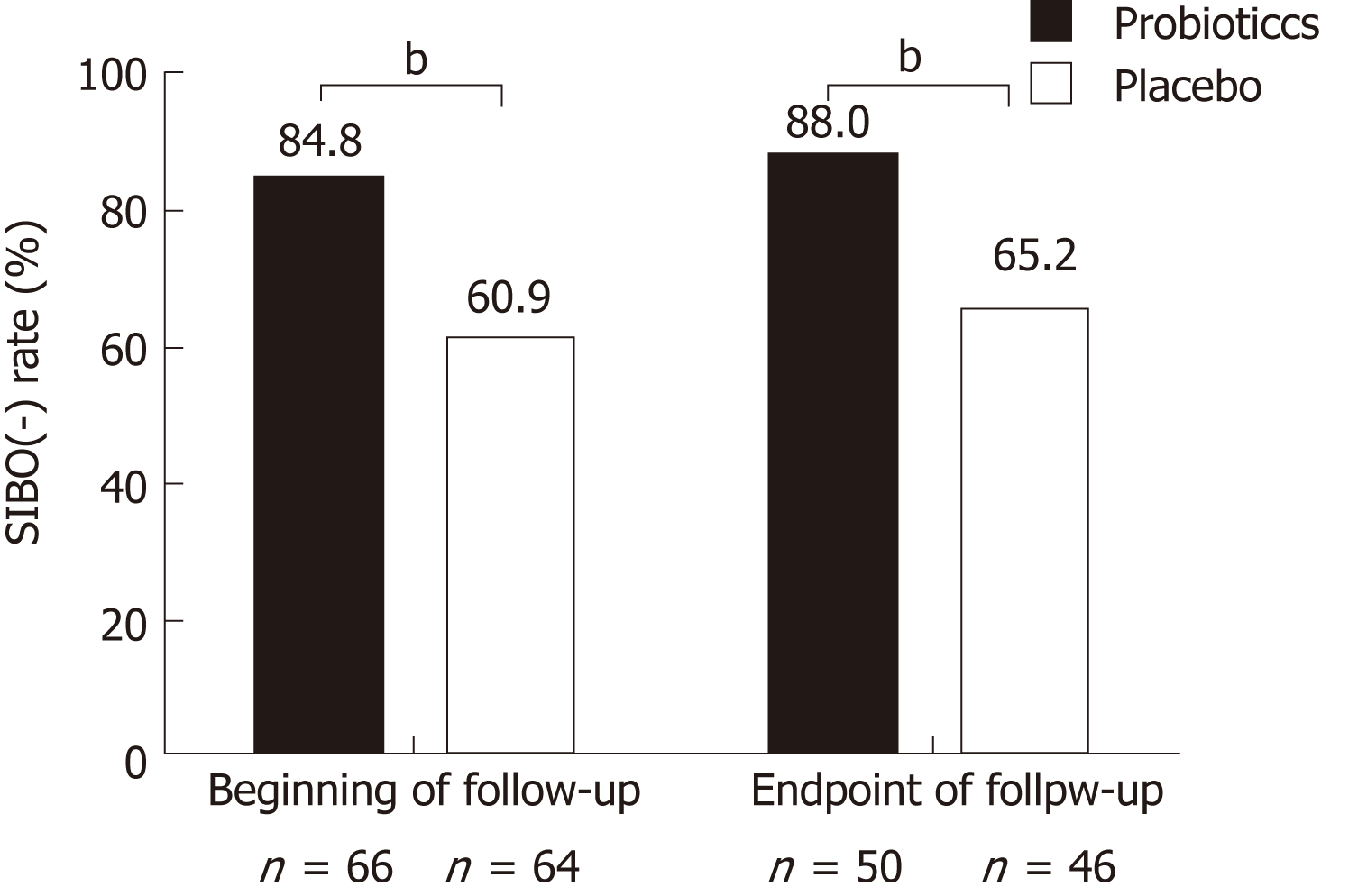
Figure 5 Proportion of patients without small intestinal bacterial overgrowth at the beginning and endpoint of follow-up.
Probiotics refers to esomeprazole 20 mg b.i.d. and live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules 500 mg t.i.d. treatment; placebo refers to esomeprazole 20 mg b.i.d. and placebo treatment. aP < 0.05; bP < 0.01; cP < 0.001. SIBO: Small intestinal bacterial overgrowth.
- Citation: Sun QH, Wang HY, Sun SD, Zhang X, Zhang H. Beneficial effect of probiotics supplements in reflux esophagitis treated with esomeprazole: A randomized controlled trial. World J Gastroenterol 2019; 25(17): 2110-2121
- URL: https://www.wjgnet.com/1007-9327/full/v25/i17/2110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i17.2110