Published online May 7, 2019. doi: 10.3748/wjg.v25.i17.2045
Peer-review started: January 21, 2019
First decision: February 21, 2019
Revised: March 26, 2019
Accepted: April 10, 2019
Article in press: April 11, 2019
Published online: May 7, 2019
Barrett’s esophagus (BE) is a change in the esophageal lining and is known to be the major precursor lesion for most cases of esophageal adenocarcinoma (EAC). Despite an understanding of its association with BE for many years and the falling incidence rates of squamous cell carcinoma of the esophagus, the incidence for EAC continues to rise exponentially. In association with this rising incidence, if the delay in diagnosis of EAC occurs after the onset of symptoms, then the mortality at 5 years is greater than 80%. Appropriate diagnosis and surveillance strategies are therefore vital for BE. Multiple novel optical technologies and other advanced approaches are being utilized to assist in making screening and surveillance more cost effective. We review the current guidelines and evolving techniques that are currently being evaluated.
Core tip: Appropriate screening and diagnosis of Barrett’s esophagus and dysplasia and thereby cancer prevention is challenging. Newer imaging modalities aid and complement the role of traditional endoscopy with biopsy. Research in this area is promising and primarily focused on improved optical technology and advanced sampling techniques.
- Citation: Steele D, Baig KKK, Peter S. Evolving screening and surveillance techniques for Barrett's esophagus. World J Gastroenterol 2019; 25(17): 2045-2057
- URL: https://www.wjgnet.com/1007-9327/full/v25/i17/2045.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i17.2045
Barrett's esophagus (BE) is characterized by the replacement of squamous epithelium normally found in the esophagus with metaplastic columnar epithelium. As a result, the proximal level of the squamocolumnar junction, also known as the z-line, no longer corresponds with the gastro-esophageal junction (GEJ). This change is a result of chronic exposure of the normal squamous epithelium to refluxed gastric material and is believed to increase the risk of evolution to neoplasia[1].
The index examination is essential for identifying and diagnosing BE as this ultimately determines follow up intervals moving forward. Using white light endoscopy (WLE), BE can be accurately visualized and then divided into short segment (shorter than 3 cm) and long segment (longer than 3 cm). Endoscopically, BE is then further graded using Prague C and M criteria. The Prague C and M criteria is a validated grading system that assesses the presence and extent of BE by measuring the circumferential (C) length and maximum (M) length of BE visualized above the GEJ[1]. Once BE has been recognized and graded, biopsies are then obtained using the Seattle protocol. The Seattle protocol is a technique that aims to identify BE with or without dysplasia and neoplasia by obtaining 4-quadrant biopsies every 1-2 centimeters within this area of identified BE. In addition to Seattle protocol, biopsies are obtained of any areas of mucosal irregularity. These biopsies are then sent for pathology where the diagnosis of BE or is confirmed by the identification of intestinal metaplasia on biopsy[1,2]. Since dysplastic and neoplastic lesions can be dispersed throughout a segment of BE, sampling error decreases the sensitivity of random biopsies using the Seattle protocol, especially for segments longer than 3 cm[3].
While this change in the esophageal lining is largely asymptomatic, BE is known to be the major precursor lesion for most cases of esophageal adenocarcinoma (EAC). Esophageal cancer is the eighth most common cancer in the world with approximately 10000 new cases of EAC diagnosed every year. Despite an understanding of its association with BE for many years and the falling incidence rates of squamous cell carcinoma of the esophagus, the incidence for EAC continues to rise exponentially[4]. In association with this rising incidence, if the delay in diagnosis of EAC occurs after the onset of symptoms, then the mortality at 5 years is greater than 80%. Alternatively, if EAC is diagnosed at an early stage, T1a, then the 5-year mortality is significantly better at greater than 80%[5]. Given this rising incidence and poor prognosis from EAC which has a known precursor lesion in BE that can be endoscopically monitored, significant interest has been placed in finding an effective way to accurately screen for BE.
Whereas there is significant concern for the rising incidence in EAC, screening is limited to a very specific patient population. Some of the limitations to screening the general population include the lack of an accurate, widely applicable risk assessment tool, lack of a cost-effect screening method and the absence of a beneficial effect on mortality. Additionally, the incidence of EAC is rising, the absolute risk of developing EAC in the setting of having BE remains low. The most recent data has shown the prevalence of BE in the general population to be around 1%-2% and the annual risk of BE converting to EAC between 0.12%-0.5%[4]. For these reasons, screening the general population for BE by endoscopic or non-endoscopic methods is not advocated. Although screening the general population may not be recommended at this time, screening targeted populations is encouraged.
While recommendations amongst the major gastroenterological societies differ, as outlined in Table 1, the overall consensus is to screen individuals with multiple risk factors for BE/EAC, and in the case of the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy to screen patients who have been experiencing symptoms for a prolonged period of time[5]. An international consensus statement (BOB CAT) recommended endoscopic screening for men older than 60 years of age who have experienced chronic gastroesophageal reflux disease (GERD) symptoms for 10 years or longer[4].
| Society (year published) | Target populations |
| American College of Gastroenterology (2016) | Primary: Male patients with either > 5 years of GERD or with more than weekly GERD symptoms and at least two other risk factors including: (1) Age > 50; (2) central obesity; (3) smoking history; (4) Caucasian; (5) first degree relative with BE or EAC |
| American Society for Gastrointestinal Endoscopy (2012) | Patients with multiple risk factors including male sex, older than 50, Caucasian, family history of BE, increased duration of reflux symptoms, smoking and obesity |
| American Gastroenterological Association (2011) | Patients with multiple risk factors including male sex, older than 50, Caucasian, chronic GERD, hiatal hernia and obesity |
| British Society of Gastroenterology (2014) | Primary: Patients with GERD and at least three risk factors including male, older than 50, Caucasian, and obesity unless there is a family history of BE or EAC which would lower threshold |
Furthermore, attempts have been made to create a validated model to determine risk of progression of BE to neoplasia. One model has been the creation of the 'Progression in BE score or PIB score' which further outlined in Tables 2 and 3. This scoring system uses the risk factors identified as significant in causing the highest risk of developing high-grade dysplasia (HGD)/EAC [male sex, smoking, length of BE and baseline low-grade dysplasia (LGD)] to determine patients with BE who are at low, intermediate and high risk for HGD or EAC. Using the point system in Table 3, patients with 0-10 points were considered low risk for progression, patients with 11-20 were considered intermediate risk and patients with 21-30 were considered high risk[6]. The annual risk of progression was 0.13% for low, 0.73% for intermediate and 2.1% for high risk groups respectively[6]. Of note, this score is useful in patients who have been diagnosed and have established BE.
| Variable | Adjusted P value and hazard ratios (95%CI) |
| Males | P = 0.0023, HR = 3.01 (1.48-6.11) |
| Smoking | P = 0.0029, HR = 1.83 (1.23-2.71) |
| Age + 10 yr | P = 0.3055, HR = 0.96 (0.89-1.04) |
| Caucasian | P = 0.8429, HR = 1.06 (0.61-1.82) |
| Hiatal hernia present | P = 0.5928, HR = 1.12 (0.73-1.72) |
| Visible lesion at baseline | P = 0.9254, HR = 1.04 (0.49-2.2) |
| Aspirin use | P = 0.2807, HR = 0.81 (0.56-1.18) |
| Non-steroidal anti-inflammatory drug | P = 0.5602, HR = 0.9 (0.64-1.28) |
| Proton pump inhibitor | P = 0.8197, HR = 0.9 (0.37-2.21) |
| Low grade dysplasia | P ≤ 0.0001, HR = 3.68 (2.56-5.31) |
| BE length + 1 cm increase in length | P ≤ 0.0001, HR = 1.12 (1.08-1.18) |
| Variable | Points |
| BE length in centimeters | |
| < 1 | 0 |
| 1 to < 2 | 1 |
| 2 to < 3 | 2 |
| 3 to < 4 | 3 |
| 4 to < 5 | 4 |
| 5 to < 6 | 5 |
| 6 to < 7 | 6 |
| 7 to < 8 | 7 |
| 8 to < 9 | 8 |
| 9 to < 10 | 9 |
| 10 + | 10 |
| Males | 9 |
| Smokers | 5 |
| Baseline confirmed LGD | 11 |
This targeted screening approach may assist in the diagnosis of BE/EAC in some patients, however, approximately one half of patients with EAC report never having symptoms of heartburn prior to their diagnosis. In addition, the gold standard for diagnosis, upper gastrointestinal endoscopy is invasive and expensive[4]. While BE is a known precursor lesion to EAC, studies have shown that more than 90% of EAC is diagnosed in the absence of a prior diagnosis of BE. While not entirely understood, a possible explanation for this occurrence is likely related to the disease being diagnosed at a later stage after the progression of BE to dysplasia and eventually EAC has already occurred. Importantly, this shows that we are missing a significant number of at risk patients with the current screening guidelines[7].
Given all of these considerations, finding a universally accepted screening modality program for EAC remains a challenge, however, identifying the key components will increase the likelihood for success. The primary element to developing a successful screening program is finding a screening tool that is "minimally or noninvasive, cost-effective, widely applicable, safe and accurate in the diagnosis of BE"[5]. Once this has been identified, it will also be important to recognize a validated risk assessment tool to target those at risk for developing BE/EAC as well as a tool to predict those at highest risk for progression of disease. The final phase will include finding a cost-effective tool to treat dysplasia or early EAC once diagnosed by screening or surveillance[5]. Ongoing research is being performed to address these issues to allow for widespread screening and subsequently the surveillance and treatment of BE and EAC.
In this section, we will review the current and emerging techniques being used for the screening of BE.
Conventional white light endoscopy: High-definition (HD) upper gastrointestinal endoscopy is currently used as the gold standard in screening targeted populations. HD has replaced standard definition (SD) endoscopy over the last several years due to the limited sensitivity and specificity of SD[8]. The image resolution of HD utilizes more than 1 million pixels compared to just 100000-400000 with SD. This enhances the ability to visualize subtle mucosal changes to allow for more accurate biopsies of areas concerning for BE or endoscopically suspected esophageal metaplasia[9].
While HD WLE is the gold standard in screening and surveillance, as discussed before, cost remains the primary limiting factor for its use as a screening tool for the general population. The cost is multifactorial and includes the cost of the procedure, sedation, and the cost of upgrading entire endoscopy systems from SD endoscopy[8]. In addition to cost, concerns also exist regarding the missed rates of dysplastic or neoplastic lesions. In a study to evaluate the efficiency of biopsies using standard protocol, the missed rates were as high as 57%[8]. As such, advanced imaging technologies have started to emerge to enhance the screening, surveillance, and treatment of patients with BE[8].
Chromoendoscopy: Chromoendoscopy is a technique where stains are applied topically to enhance mucosal visualization during upper endoscopy. The goal of this approach is to improve visualization of the mucosa and the vascular pattern of absorption to improve detection of abnormalities and target biopsies[9]. The most commonly used stains include indigo carmine, methylene blue, and acetic acid[1,4].
Indigo carmine is a non-absorptive contrast dye frequently used in conjunction with magnification endoscopy to identify irregular mucosa and pit patterns seen within segments of BE[1,9]. These mucosal findings have been shown to correlate with presence of intestinal metaplasia and dysplasia. Methylene blue is a chemical that can be absorbed by intestinal epithelium without being absorbed by squamous or gastric epithelium. When compared to traditional surveillance techniques, several studies have shown methylene blue could discern areas of intestinal metaplasia and dysplasia with high accuracy and fewer biopsies[9]. Despite these studies, overall, traditional techniques have been shown to be non-inferior which in conjunction with a potential risk for carcinogenesis from methylene blue, the widespread use of chromoendoscopy using methylene blue has been limited[9].
Acetic acid in combination with either HD or magnification endoscopy works to provide contrast enhancement of the mucosa. Initial application of acetic acid turns all of the esophageal and gastric mucosa white. After several minutes, however, while the normal mucosa remains white, gastric columnar mucosa and areas of BE will turn red[1]. Multiple studies looking at patients undergoing surveillance for BE have shown that targeted biopsies with acetic acid chromoendoscopy yield higher rates of detection of dysplasia and neoplasia while fewer biopsies are required[1].
Lugol's solution or more commonly known as Lugol’s Iodine (LI) is a compound stain that contains iodine and potassium iodide that when absorbed by the squamous mucosa of the esophagus, stains it brown. By staining the squamous epithelium brown, LI highlights any metaplastic columnar epithelium within the esophagus[10,11]. One small study evaluated the accuracy of diagnosing BE using LI in 11 subjects with known columnar epithelium in the esophagus and compared them with 12 control subjects. The sensitivity and specificity of of diagnosing BE using LI was 89% and 93% respectively[12].
Overall, the advantage of chromoendoscopy is that it is relatively inexpensive and the chemical solutions are readily available for use. There are however several disadvantages to using chromoendoscopy. The biggest disadvantage is high inter-observer variability in ability to identify abnormal mucosa. Additionally, chromoendoscopy can be labor intensive requiring a separate catheter and often multiple sprays in order to adequately visualize the mucosa[1,4,9].
Electronic chromoendoscopy: Electronic chromoendoscopy generally refers to endoscopic imaging technologies that enhance the mucosal surface and blood vessels through contrast enhancement[1]. This section will review four processor enhanced electronic systems: Narrow band imaging (NBI), Fuji Intelligent Chromoendoscopy (FICE), i-SCAN, and blue light imaging (BLI).
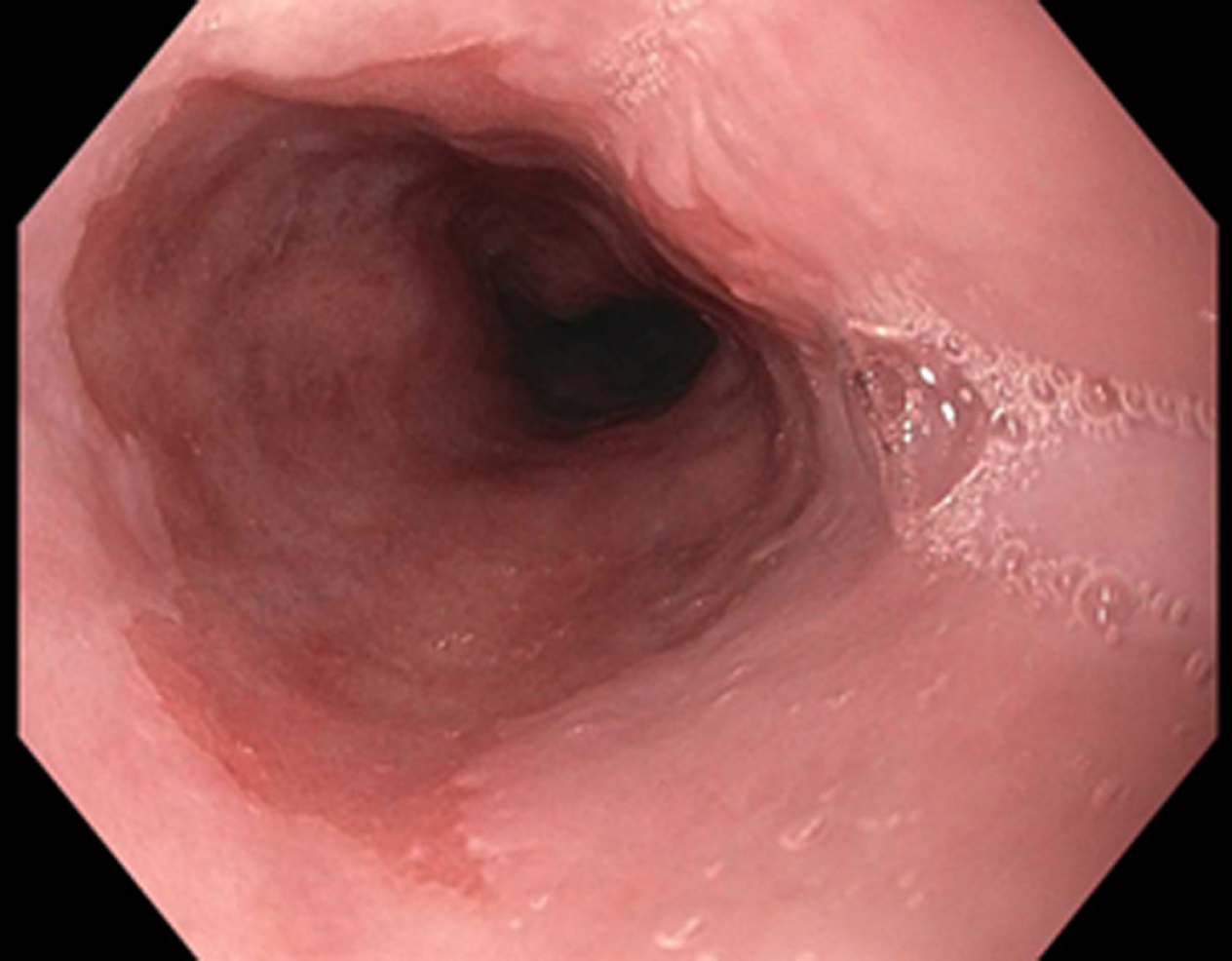
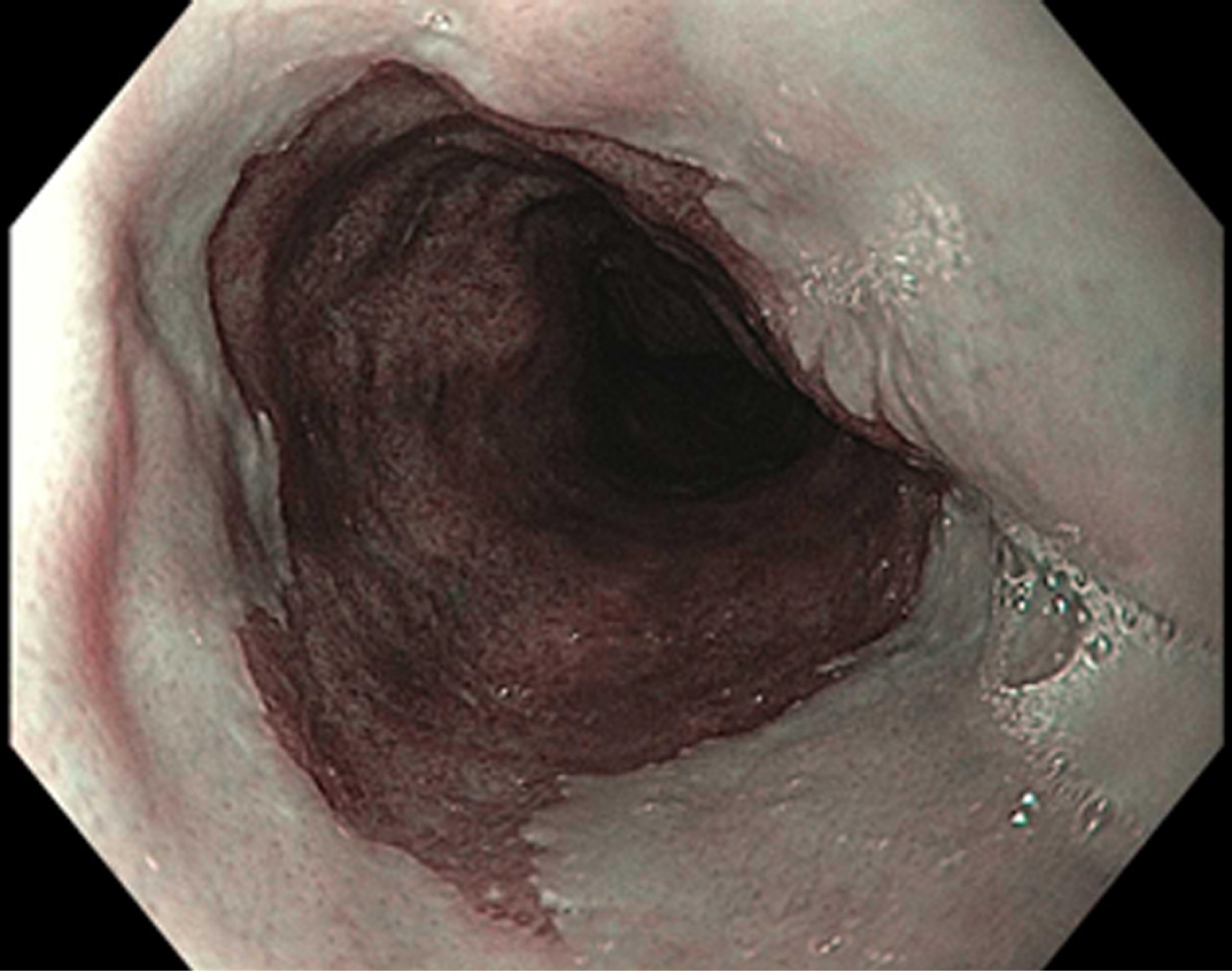
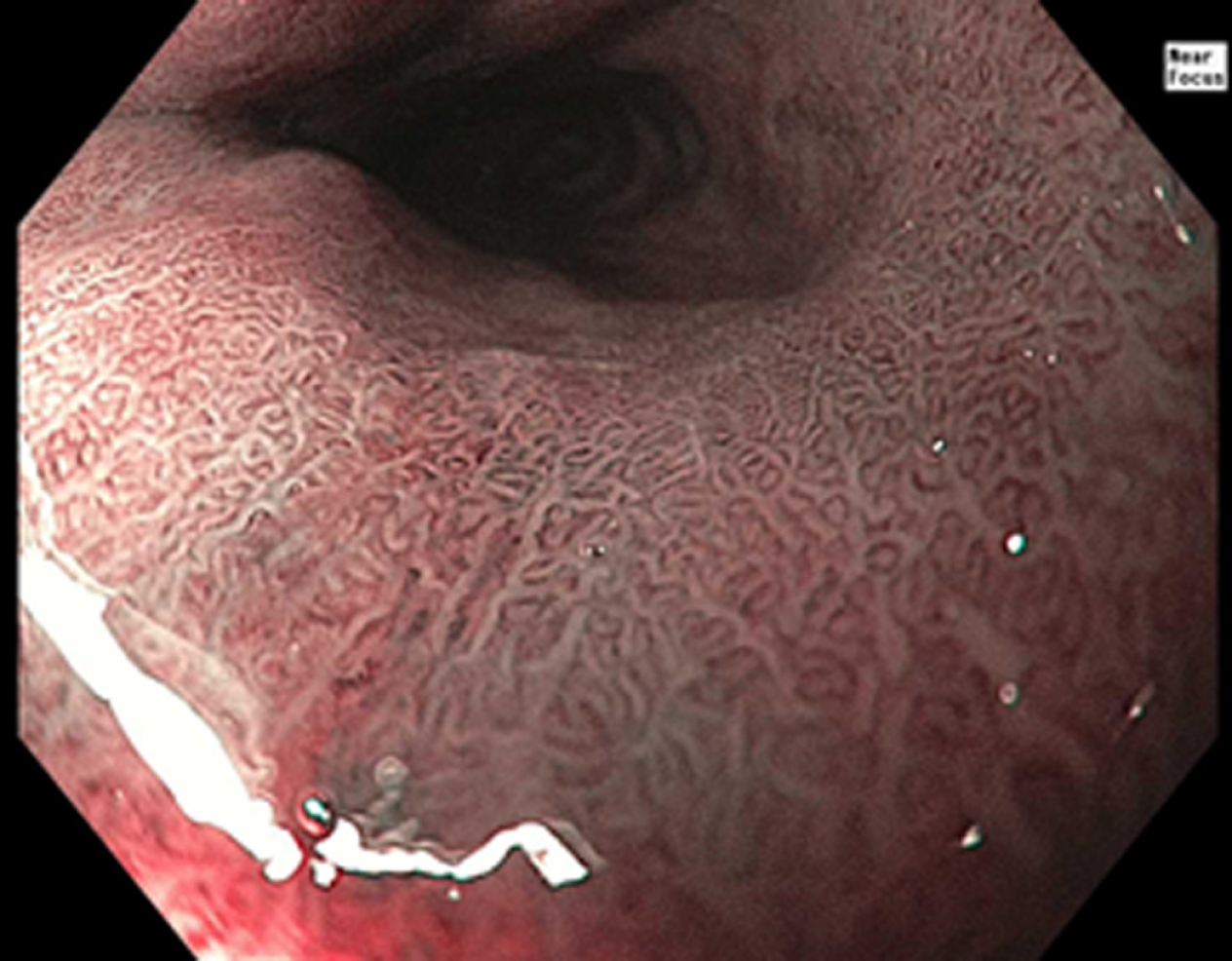
NBI (Olympus Evis Exera System®) differs from chromoendoscopy in that no stains are used. Instead, NBI works by enhancing the resolution of the mucosal surface by restricting the range of wavelengths of light. Several meta-analysis studies have shown NBI to do well in detecting HGD with high sensitivity (96%) and specificity (94%) in one study while using fewer biopsies when compared to WLE. At the same time, several studies have been unable to show a difference in detecting neoplasia when compared to WLE[4,9] (Figures 1-3). The advantages to NBI are that it is relatively cheap; widely available given it is integrated in most standard equipment and its ease of use[9]. NBI also has the advantage over dye-based chromoendoscopy because there is no risk for potential toxicity[4]. Previously, a disadvantage of NBI has been the lack of a universal classification system; however, in 2016, the Barrett's International NBI Group aimed to develop and validate a classification system to identify dysplasia and EAC in patients with BE using NBI. The BING criteria were created by a group of experts in NBI who reviewed images of non-dysplastic BE, dysplastic BE, and EAC to characterize the different mucosal and vascular patterns using NBI (Table 4). Using these criteria, patients undergoing surveillance/treatment of BE were then recruited to obtain high resolution NBI images and biopsies for histologic review. Using the newly formed BING criteria, the NBI images from these patients were reviewed by experts to determine the validity of the BING criteria for accuracy. The results from this study found that the BING criteria identified patients with dysplasia with an overall accuracy of 85% and when dysplasia identified with a high degree of confidence, the overall accuracy was 92% with a high level of inter-observer agreement[13].
| Mucosal pattern | |
| Circular, ridged/villous, or tubular | Regular |
| Absent or irregular | Irregular |
| Vascular pattern | |
| Blood vessels situated regularly along or between mucosal ridges and/or showing normal long branching patterns | Regular |
| Focally or diffusely distributed vessels not following normal architecture of the mucosa | Irregular |
FICE (FUJIFILM Endoscopy System®) manipulates certain ranges of wavelengths (red, green and blue) to yield a color-enhanced image of the superficial mucosa and vascular structures in real time. A study comparing FICE to acetic acid chromoendoscopy found FICE to have comparable sensitivity in detecting neoplasia in BE[9]. At this time, however, more data is needed to assess the right setting for tissue diagnosis in FICE.
Similar to FICE, i-SCAN (PENTAX Endoscopy System®) is software driven electronic chromoendoscopy technique that manipulates wavelengths to produce an enhanced image. Limited data also exists for i-SCAN. In a randomized trial comparing standard protocol biopsies with i-SCAN or acetic acid chromoendoscopy, use of i-SCAN was comparable to acetic acid and superior to random biopsies in diagnosing intestinal metaplasia[9]. More data is needed to assess the right setting for tissue diagnosis for i-SCAN.
BLI or endoscopy (FUJIFILM ELUXEO 7000®) is a high quality optical technology that aims to provide enhanced visualization and differentiation of mucosal surfaces and vessel structures. BLI is felt to assist in better identifying changes in surface relief, defined as subtle elevations and depressions relative to normal surrounding flat mucosa. A 2018 study aimed to evaluate the additional value BLI could provide over WLE for identifying BE neoplasia[14]. Findings from this study showed that BLI added value to WLE for visualization of BE neoplasia and that experts appreciated BLI more than WLE for the delineation of BE neoplasia especially in lesions that were difficult to delineate with WLE alone[14].
Auto fluorescence imaging: Mucosa contains endogenous tissue fluorophores, which are biological structures that emit fluorescent light when exposed to light of a shorter wavelength. Auto flourescence imaging (AFI) operates on the principle that mucosa differs in the fluorescence it admits based on the type of tissue. For instance, while normal mucosa appears green under fluorescence excitation, dysplasia and neoplasia appears "magenta or purple"[9]. Using these principles, AFI can detect and characterize changes in mucosa.
Several early studies have shown AFI has good sensitivity increasing the detection of HGD and early neoplasia, however, specificity is poor with a high false positive rate[4,9]. Endoscopic trimodal imaging (ETMI) combines AFI with WLE and NBI in an attempt to maintain the sensitivity and reduce the false positive rate seen with AFI alone. Despite lowering the false positive rates, several studies have been unable to show a difference in detection rates between ETMI with targeted biopsies and standard endoscopy with random biopsies[9]. While AFI may be helpful as an adjunct to WLE, due to the high false positive rates, AFI alone is not an adequate replacement for current guideline recommendations.
Microscopic endoscopy: In conjunction with WLE and other advanced endoscopic imaging techniques, microscopic endoscopy allows for a real-time histological assessment of the esophageal mucosa during endoscopy[9].
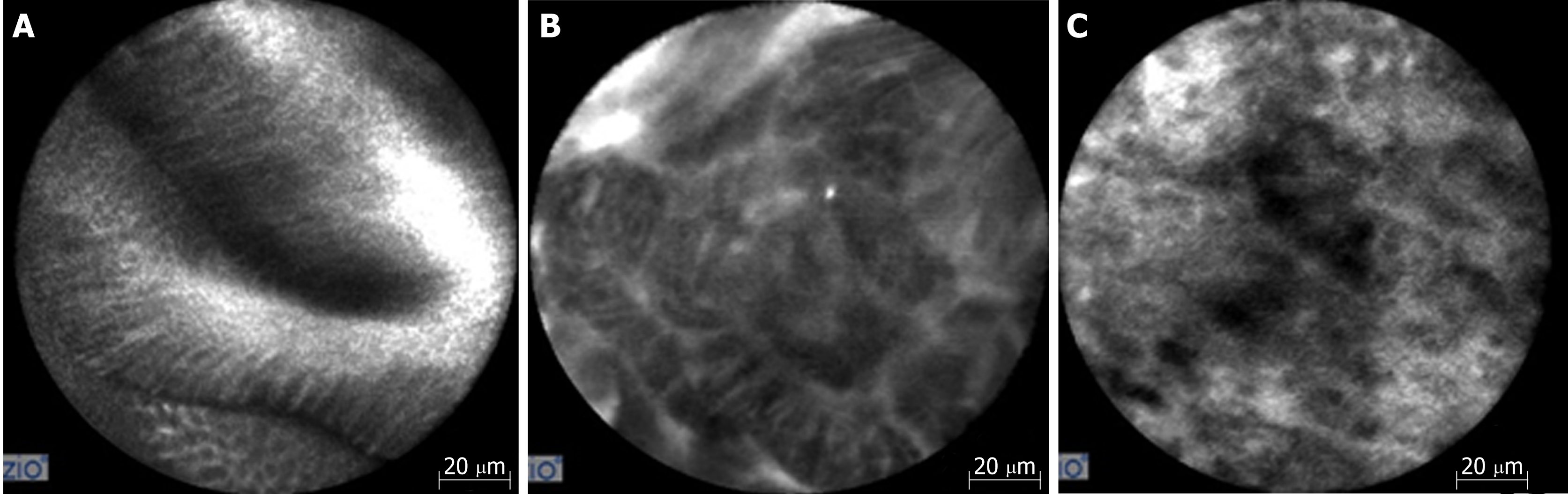
Confocal laser endomicroscopy (CLE) is an advanced imaging technique that can magnify mucosa up to 1000 times to acquire submucosal images up to 250 micrometers below the mucosal surface. Most CLE studies have been performed using either endoscopic CLE (eCLE) where a confocal microscope is placed in to the tip of an endoscope or probe-based CLE (pCLE) where a probe can be introduced through an accessory channel. Given that premalignant lesions such as BE with dysplasia are challenging to identify with conventional screening, both eCLE and pCLE use a blue laser light and a fluorescent to enhance mucosal structures that are vascular-supplied[4,8-9,15] (Figure 4).
One approach to improve detection has been to develop a peptide to better find molecular changes. In particular, a fluorescently labeled peptide has been developed that specifically binds to HGD and EAC. In a 2013 study to evaluate the validity of this approach, after topically applying the peptide to the esophagus, confocal endomicroscopy was performed. In cases of esophageal neoplasia, the results showed a 3.8 fold greater fluorescent intensity compared to BE and normal squamous epithelium. The sensitivity of which was 75% and specificity was 97%. Additionally, the peptide is felt to be safe, with no toxicity in animal or patient studies[16].
Studies have shown an advantage in using CLE compared to WLE for detecting HGD and EAC and reducing the number of biopsies required to make a diagnosis. Additionally, pCLE has a widely accepted classification criteria called the Miami criteria, reviewed in Table 5, that has been validated in random controlled trials[17]. One major concern regarding CLE is that all studies assessing its potential use were performed by expert endoscopists at "tertiary referral centers" where a higher percentage of patients with dysplasia are likely to be located[4]. Concerns over the practical use of CLE as a primary screening tool also exist due to high equipment costs, prolonged procedure time, and the training required using the equipment and interpreting images.
| Histology | Confocal characteristics |
| 1 Normal Squamous Epithelium | Flat Cells with bright intrapapillary capillary loops |
| 2 Non-dysplastic Barrett's Esophagus | Uniformed villiform architecture with dark goblet cells |
| 3 Barrett's esophagus with high-grade dysplasia | Villiform structures with dark, irregular and thick borders |
| 4 Adenocarcinoma | Disorganized villiform architecture and dilated irregular vessels |
Endocytoscopy uses WLE and special magnification lenses to allow microscopic evaluation of the mucosa. Similar to dye-based chromoendoscopy, a contrast agent, usually methylene blue is applied to the surface of the mucosa, then depending on the system used, magnification can be up to 1400-fold of normal[9]. Studies have been performed to evaluate effectiveness in diagnosing squamous esophageal cancer and dysplasia and results have been variable. Currently, Endocytoscopy is not universally used for evaluation in patients with BE[9]. Overall, Endocytoscopy has shown promise in identifying dysplastic and neoplastic lesions with its primary limitation owing inability to perform wide-field screening of the mucosa. As such, one potential future application could include its use as an adjunct to other techniques to better visualize specific, targeted lesions[18].
Optical coherence tomography/volumetric laser endomicroscopy: Optical coherence tomography (OCT) is similar to ultrasound except that it uses light waves rather than sound waves to obtain subsurface, cross-sectional images of a mucosal surface. During standard endoscopy, images are obtained by introducing a catheter through the accessory channel[9,15]. One prospective clinical study assessing the presence of dysplasia in patients with BE using OCT found an 83% sensitivity and 75% specificity respectively. Several other studies have been performed to evaluate OCT and overall results have varied[9].
Optical frequency domain imaging otherwise referred to as volumetric laser endomicroscopy (VLE) is similar to OCT but allows for high resolution, high-speed acquisition of larger areas of the mucosal surface. In practice, VLE can be used to screen for BE, for surveillance of BE and for mapping prior to ablation or endoscopic resection similar to other advanced imaging technology. VLE also has the ability unlike other technology to evaluate for residual BE below neosquamous mucosa after endoscopic therapy[15]. Studies are now starting to focus on obtaining interobserver agreement regarding image interpretation and correlating images with histology[9].
Tethered capsule endomicroscopy: Tethered capsule endoscopy (TCE) is a new device that obtains images evaluating for BE by utilizing the imaging capabilities of OCT through the use of a tethered capsule. The pilot study performed in 2012 to test the overall safety and acceptability of the TCE device resulted in no adverse events and 89% of patients able to swallow the capsule. Additionally, of the patients tested, 62% recorded they would prefer TCE to endoscopy[19]. Another study using TCE evaluated 17 participants with suspicion or confirmed BE. Of the 17 patients, 13 had an esophagogastroduodenoscopy (EGD) within 12 mo of TCE and were able to swallow the capsule[19]. A blinded comparison of Prague C and M criteria for BE in TCE vs EGD was performed. Findings showed a strong to very strong correlation (r = 0.7-0.83, P < 0.5) for circumferential (C) extent and a strong correlation (r = 0.77-0.78, P < 0.01) for maximum (M) extent of BE[19].
Spectroscopy: Spectroscopy uses variation in scattered light across a full spectrum to obtain information on crowding, vascularity, size and tissue structure[9]. Raman spectroscopy specifically detects scattered light that has been changed in wavelength and creates characteristic peaks that correspond to normal vs abnormal mucosa. Early studies have shown good success in real-time detection of BE and neoplasia.
Wide area transepithelial sampling with 3-dimensional tissue analysis: Wide area transepithelial sampling with computer 3-dimensional analysis (WATS-3D) is a new technique for screening and surveillance of BE. WATS-3D is able to obtain transepithelial specimens of BE by using a unique abrasive brushing instrument. The samples of tissue are then analyzed through a high-speed computer system to find the most suspicious cells which can then be reviewed by a pathologist[1,4,20].
In a multicenter prospective randomized trial that included 160 patients with BE, WATS-3D plus Seattle protocol was compared to Seattle protocol alone to determine if the combination protocol could improve the detection of dysplasia and neoplasia. In this study, Seattle protocol alone detected only 7 cases of HGD and neoplasia. With the addition of WATS-3D, an additional 23 cases of HGD and neoplasia were detected that were not found using Seattle protocol alone[1,4].
A second, larger prospective trial was performed that evaluated more than 4000 patients with suspected or established BE[1]. Patient either underwent EGD with Seattle protocol biopsies alone or Seattle protocol plus WATS-3D. In the group that underwent the protocol alone, BE was detected in 594 patients vs 799 patients tested by WATS-3D. Of the 799 patients diagnosed with BE by WATS-3D, 493 of these patients were not diagnosed with BE by Seattle protocol. Unique to this study was the evaluation for LGD. In the group tested with WATS-3D, 33 patients were diagnosed with LGD. Of these 33 patients, 23 had negative results for LGD by Seattle protocol alone[1]. Early results have been promising for the potential implementation of WATS-3D to improve efficiency for BE surveillance or possibly even screening however more research is required to determine its generalizability for wide-spread use.
Cytosponge™: Cytosponge™ (Medtronic, Menneapolis, MN, United States) is a novel device that consists of an ingestible gelatin capsule on a string. Once the device makes it to the stomach, the capsule dissolves and a small sponge is revealed that can then be withdrawn through the esophagus and out of the mouth by pulling the string. During this process, the sponge is able to collect esophageal cells to screen for different disease processes like BE dysplasia, and esophageal carcinoma. Once the cells are collected, the sponge is then tested to evaluate for trefoil factor 3 (TFF3) which is a biomarker for BE. Identification of this biomarker helps to distinguish BE from gastric cells and squamous cells within the esophagus[1].
Several prospective trials have been performed to evaluate the accuracy of the Cytosponge™ TFF3 test in screening for BE. The BE Screening Trial 1 (BEST1) cohort study looked at 501 patients with previous prescriptions for acid suppression[1]. Testing with Cytosponge™ with TFF3 showed 73% sensitivity and 94% specificity for patients with short segment BE which improved to 90% sensitivity and 93.5% specificity for long segment BE. The BE Screening Trial 2 (BEST2) subsequently evaluated 1110 patients with Cytosponge™ and endoscopy[1]. Findings from this trial yielded a sensitivity of 80% and specificity of 92% for short segment BE. Sensitivity increased to 87% in those with long segment BE[1].
In regards to safety, a multicenter review of 5 prospective trials using Cytosponge™ was published in August 2018[21]. Of 2672 Cytosponge™ procedures across these five trials, only two adverse events occurred related to the device. The adverse events included a single case of minor pharyngeal bleeding and a single case of device detachment. Additionally this study showed that patients tolerated the device well with > 90% achieving a successful swallow on the first attempt [21].
The Cytosponge™ offers the convenience of administration in addition to a cost effective alternative to traditional techniques. A recent study compared the cost-effectiveness of Cytosponge™ followed by endoscopic treatment to endoscopic screening followed by endoscopic treatment and found Cytosponge™ screening followed by endoscopic treatment to be more cost effective[22].
Transnasal endoscopy: Transnasal endoscopy (TNE) is a screening technique where a thin endoscope is introduced through the nares into the esophagus to evaluate for BE. TNE can be performed either in a hospital (hTNE) or mobile/outpatient (mTNE) setting and can be performed using only topical anesthetic without the need for sedation[1].
In a prospective study, 121 patients with either GERD symptoms or known BE were randomized to either transnasal endoscopy followed by standard endoscopy or standard endoscopy followed by transnasal endoscopy. The prevalence of BE in the two groups showed no significant difference at 26% and 30%, respectively (P value 0.503). Several other studies have demonstrated similar findings as well as better overall tolerance of transnasal endoscopy compared to standard endoscopy[1].
Similar to Cytosponge™, transnasal endoscopy is both easily tolerated and offers cost-effectiveness compared to standard endoscopy. In addition to reducing cost by eliminating the need for sedation, a new device called transnasal endosheath endoscopy (TNE-5000 with EndoSheath Technology, Vision Sciences, Inc., New York, NY, United States) utilizes a reusable endoscope with a disposable outer sterile sheath[1]. Overall, findings from studies involving transnasal endoscopy have shown promise as a potential future screening tool for BE.
Biomarker panels: Finding potential biomarkers for BE is a robust and exciting area of research. While several biomarkers for BE in the areas of dysplasia, genome markers, and gene expression alterations have been discovered, a single, ideal biomarker for BE has yet be identified[23].
One biomarker that has been proposed and shown promise as an adjunct to a traditional biopsy approach is immunostaining p53. This tumor suppressor gene becomes activated by injury to DNA to decrease cell multiplication to allow time for DNA repair and thus prevent damaged cells from replicating[24]. If the injury is too severe, then p53 can provoke cell death via apoptosis. One of the sentinel events that occurs in the progression of BE to neoplasia is the inactivation of p53. Given this occurrence, several studies are looking at p53 expression as a biomarker to determine risk for progression from BE to dysplasia and ultimately EAC[24]. Recently, a prospective study evaluated aberrant p53 expression to predict progression to HGD or EAC. Of 91 subjects with BE without dysplasia initially, 11 progressed to HGD or EAC. Aberrant p53 expression was evaluated in all of the subjects and was found significantly more often in those who developed HGD or EAC (63.6%) compared to subjects did not progress (7.5%)[25].
Another recent study assessed multiple proposed biomarkers in a case-control study to predict the progression of BE to EAC[26]. In this study, 130 patients with BE who progressed to HGD and/or EAC were compared with 130 patients with BE who never progressed. Using abnormal DNA, P53, Cyclin A, and Aspergillus oryzae lectin (AOL) in routine paraffin embedded biopsies sections, conditional logistic regression analysis was used on this patient population to estimate an odds ratio of progression. Findings from this study showed that of these biomarkers, expert LGD, AOL, and p53 all independently predicted progression of BE to neoplasia[26]. While research in this area is ongoing, early findings offer promise at identifying a tool to target more aggressive surveillance and treatment strategies in patients with BE and potentially an improved method for screening in the future.
Breath testing using an electronic nose device: Electronic nose (e-nose) devices have been invented to utilize chemical to electrical interfaces to measure subtle differences in volatile organic compounds (VOCs). When combined with a machine-learning program, identification of these VOCs can be used as a noninvasive diagnostic test to differentiate certain disease states[27].
A recent cross-sectional study using this technology was performed on a group of 122 patients with a history of dysplastic BE to evaluate for the presence or absence of BE. Each subject provided a 5-min breathing sample in a fasting state prior to undergoing an upper endoscopy with biopsies. Using E-nose technology to categorize patients with findings characteristic of BE, detection of BE was found to have a sensitivity of 82% and specificity of 80%[27]. Future studies will be need to compare patients without BE but given its ease of use and portability, e-nose could be a potential screening tool for BE in the future.
In conclusion, as mentioned before, the incidence of EAC is rising. Given its poor prognosis, especially in the setting of having a known precursor lesion in BE that can be endoscopically monitored, identifying an efficient, cost-effective way to accurately screen for BE has become increasingly important. Research in this area is promising and primarily has focused on improved optical technology and advanced sampling techniques. The current techniques along with their advantages and disadvantages are listed below in Table 6. While promising in multiple areas, further research is required before a designated screening tool for BE can be universally implemented.
| Advantage | Disadvantage | |
| Standard definition white light endoscopy | Provides wide-field imaging and is widely available | Decreased sensitivity when compared to high definition |
| High definition white light endoscopy | Provides wide-field imaging and is widely available with improved image quality | Cost of procedure, sedation and in some cases updating entire endoscopy system. Some concerns over missed rates of dysplastic lesions |
| Dye-based chromoendoscopy | Provides wide-field imaging with benefit of mucosal enhancement | Additional steps in procedure are time consuming and some concerns over harm of contrast |
| Narrow band imaging | Provides wide-field imaging and is widely available with improved sensitivity and without need for contrast. Relatively cheap. | Still requires white light endoscopy as an adjunct with unclear evidence on its benefits when compared to white light endoscopy alone |
| Flexible intelligent chromoendoscopy and i-SCAN | Provides wide field imaging without the need for contrast | Not widely available and not enough research to determine benefits compared to standard of car |
| Blue light imaging | Helpful in defining subtle changes in elevation and depression of the mucosa | Beneficial as an adjunct to WLE only and hence requires similar costs. Not widely available. |
| Auto flourescence imaging | Provides wide field imaging with improved sensitivity and without the need for contrast | Poor specificity with high false positive rate. |
| Confocal laser endomicroscopy | Provides in vivo information, has a validated scoring classification, and can be used with any endoscope | Does not provide wide-field imaging, requires fluorescein prolonging procedure time, requires expert interpretation and expensive |
| Endocytoscopy | Increases ability to identify dysplastic and neoplastic lesions | Does not provide wide-field imaging and requires giving contrast agent |
| Optical coherence tomography | Provides in vivo information without need for contrast or fluorescein. Ability to evaluate subsurface | Does not provide wide-field imaging and research has varied and is ongoing |
| Volumetric laser endomicroscopy | Similar to OCT but provides high resolution, high speed images over wider surface area | Expensive and studies are still working to obtain interobserver agreement and correlating images with histology |
| Tethered capsule endomicroscopy | Utilizes same technology used for OCT and is safe, well tolerated by patients | Early in stages of research |
| Spectroscopy | Early studies have shown good success in real time detection of BE and neoplasia | Early in stages of research |
| wide area transepithelial sampling | Provides wide area sampling of tissue with high sensitivity and specificity and easy to use | Not yet widely available? Regarding cost and more research needed |
| Cytosponge | Generally safe and well tolerated with low cost | Still requires endoscopy for treatment if abnormality is identified |
| Transnasal Endoscopy | Generally safe and well tolerated with relatively lower cost than endoscopy without the need for general sedation. Can be used in clinic as well as hospital | While early studies have shown equivocal ability to diagnosis BE compared to conventional endoscopy, more research required |
| Biomarker panels | Early studies have shown ability to predict progression of BE from non-dysplastic to neoplasia | A single, ideal biomarker has not been delineated and more research is required. |
| Breath testing with an electronic nose device | Safe and well tolerated and easy to use with overall cost-effectiveness | Sensitivity and specificity are good but not great compared to some other methods and research at this point is limited |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Espinel J, Herbella F, Kariv R, Lei YC, Mastracci L, Savarino V, Shiryajev YN S-Editor: Yan JP L-Editor:A E-Editor: Ma YJ
| 1. | Komatsu Y, Newhams KM, Jobe BA. Enhancing the Detection of Barrett Esophagus. Thorac Surg Clin. 2018;28:453-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | ASGE Technology Committee. Thosani N, Abu Dayyeh BK, Sharma P, Aslanian HR, Enestvedt BK, Komanduri S, Manfredi M, Navaneethan U, Maple JT, Pannala R, Parsi MA, Smith ZL, Sullivan SA, Banerjee S. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett's esophagus. Gastrointest Endosc. 2016;83:684-98.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Falk GW, Rice TW, Goldblum JR, Richter JE. Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 1999;49:170-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Maes S, Sharma P, Bisschops R. Review: Surveillance of patients with Barrett oesophagus. Best Pract Res Clin Gastroenterol. 2016;30:901-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Blevins CH, Iyer PG. Who Deserves Endoscopic Screening for Esophageal Neoplasia? Gastrointest Endosc Clin N Am. 2017;27:365-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Parasa S, Vennalaganti S, Gaddam S, Vennalaganti P, Young P, Gupta N, Thota P, Cash B, Mathur S, Sampliner R, Moawad F, Lieberman D, Bansal A, Kennedy KF, Vargo J, Falk G, Spaander M, Bruno M, Sharma P. Development and Validation of a Model to Determine Risk of Progression of Barrett's Esophagus to Neoplasia. Gastroenterology. 2018;154:1282-1289.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 985] [Cited by in F6Publishing: 921] [Article Influence: 70.8] [Reference Citation Analysis (1)] |
| 8. | Kandel P, Wallace MB. The Role of Adjunct Imaging in Endoscopic Detection of Dysplasia in Barrett's Esophagus. Gastrointest Endosc Clin N Am. 2017;27:423-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Naveed M, Dunbar KB. Endoscopic imaging of Barrett's esophagus. World J Gastrointest Endosc. 2016;8:259-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 10. | Canto MI. Vital staining and Barrett's esophagus. Gastrointest Endosc. 1999;49:S12-S16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Singh R, Mei SC, Sethi S. Advanced endoscopic imaging in Barrett's oesophagus: A review on current practice. World J Gastroenterol. 2011;17:4271-4276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Woolf GM, Riddell RH, Irvine EJ, Hunt RH. A study to examine agreement between endoscopy and histology for the diagnosis of columnar lined (Barrett's) esophagus. Gastrointest Endosc. 1989;35:541-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Sharma P, Bergman JJ, Goda K, Kato M, Messmann H, Alsop BR, Gupta N, Vennalaganti P, Hall M, Konda V, Koons A, Penner O, Goldblum JR, Waxman I. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett's Esophagus Using Narrow-Band Imaging. Gastroenterology. 2016;150:591-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | de Groof AJ, Swager A, Pouw RE, Weusten BLAM, Schoon EJ, Bisschops R, Pech O, Meining A, Neuhaus H, Curvers WL, Bergman JJGHM. Blue-light imaging has an additional value to white-light endoscopy in visualization of early Barrett's neoplasia: an international multicenter cohort study. Gastrointest Endosc. 2018;89:749-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Zihni AM, DeMeester SR. Advanced Endoluminal Technologies for Barrett's Esophagus: Focus on Optical Coherence Tomography and Confocal Laser Endomicroscopy. Thorac Surg Clin. 2018;28:465-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Sturm MB, Piraka C, Elmunzer BJ, Kwon RS, Joshi BP, Appelman HD, Turgeon DK, Wang TD. In vivo molecular imaging of Barrett's esophagus with confocal laser endomicroscopy. Gastroenterology. 2013;145:56-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Leggett CL, Gorospe EC. Application of confocal laser endomicroscopy in the diagnosis and management of Barrett's esophagus. Ann Gastroenterol. 2014;27:193-199. [PubMed] [Cited in This Article: ] |
| 18. | ASGE Technology Committee. Kwon RS, Wong Kee Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Mamula P, Pedrosa MC, Rodriguez SA, Tierney WM. Endocytoscopy. Gastrointest Endosc. 2009;70:610-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Gora MJ, Quénéhervé L, Carruth RW, Lu W, Rosenberg M, Sauk JS, Fasano A, Lauwers GY, Nishioka NS, Tearney GJ. Tethered capsule endomicroscopy for microscopic imaging of the esophagus, stomach, and duodenum without sedation in humans (with video). Gastrointest Endosc. 2018;88:830-840.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Vennalaganti PR, Kaul V, Wang KK, Falk GW, Shaheen NJ, Infantolino A, Johnson DA, Eisen G, Gerson LB, Smith MS, Iyer PG, Lightdale CJ, Schnoll-Sussman F, Gupta N, Gross SA, Abrams J, Haber GB, Chuttani R, Pleskow DK, Kothari S, Goldblum JR, Zhang Y, Sharma P. Increased detection of Barrett's esophagus-associated neoplasia using wide-area trans-epithelial sampling: a multicenter, prospective, randomized trial. Gastrointest Endosc. 2018;87:348-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Januszewicz W, Tan WK, Lehovsky K, Debiram-Beecham I, Nuckcheddy T, Moist S, Kadri S, di Pietro M, Boussioutas A, Shaheen NJ, Katzka DA, Dellon ES, Fitzgerald RC; BEST1 and BEST2 study investigators. Safety and Acceptability of Esophageal Cytosponge Cell Collection Device in a Pooled Analysis of Data From Individual Patients. Clin Gastroenterol Hepatol. 2019;17:647-656.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Heberle CR, Omidvari AH, Ali A, Kroep S, Kong CY, Inadomi JM, Rubenstein JH, Tramontano AC, Dowling EC, Hazelton WD, Luebeck EG, Lansdorp-Vogelaar I, Hur C. Cost Effectiveness of Screening Patients With Gastroesophageal Reflux Disease for Barrett's Esophagus With a Minimally Invasive Cell Sampling Device. Clin Gastroenterol Hepatol. 2017;15:1397-1404.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Qureshi AP, Stachler MD, Haque O, Odze RD. Biomarkers for Barrett's esophagus - a contemporary review. Expert Rev Mol Diagn. 2018;18:939-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Konda VJA, Souza RF. Biomarkers of Barrett's Esophagus: From the Laboratory to Clinical Practice. Dig Dis Sci. 2018;63:2070-2080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Davelaar AL, Calpe S, Lau L, Timmer MR, Visser M, Ten Kate FJ, Parikh KB, Meijer SL, Bergman JJ, Fockens P, Krishnadath KK. Aberrant TP53 detected by combining immunohistochemistry and DNA-FISH improves Barrett's esophagus progression prediction: A prospective follow-up study. Genes Chromosomes Cancer. 2015;54:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Duits LC, Lao-Sirieix P, Wolf WA, O'Donovan M, Galeano-Dalmau N, Meijer SL, Offerhaus GJA, Redman J, Crawte J, Zeki S, Pouw RE, Chak A, Shaheen NJ, Bergman JJGHM, Fitzgerald RC. A biomarker panel predicts progression of Barrett's esophagus to esophageal adenocarcinoma. Dis Esophagus. 2019;32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Chan DK, Zakko L, Visrodia KH, Leggett CL, Lutzke LS, Clemens MA, Allen JD, Anderson MA, Wang KK. Breath Testing for Barrett's Esophagus Using Exhaled Volatile Organic Compound Profiling With an Electronic Nose Device. Gastroenterology. 2017;152:24-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |