Published online Dec 7, 2018. doi: 10.3748/wjg.v24.i45.5167
Peer-review started: September 19, 2018
First decision: October 16, 2018
Revised: October 23, 2018
Accepted: November 9, 2018
Article in press: November 9, 2018
Published online: December 7, 2018
Processing time: 79 Days and 0.4 Hours
To integrate clinically significant variables related to prognosis after curative resection for gallbladder carcinoma (GBC) into a predictive nomogram.
One hundred and forty-two GBC patients who underwent curative intent surgical resection at Peking Union Medical College Hospital (PUMCH) were included. This retrospective case study was conducted at PUMCH of the Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC) in China from January 1, 2003 to January 1, 2018. The continuous variable carbohydrate antigen 19-9 (CA19-9) was converted into a categorical variable (cCA19-9) based on the normal reference range. Stages 0 to IIIA were merged into one category, while the remaining stages were grouped into another category. Pathological grade X (GX) was treated as a missing value. A multivariate Cox proportional hazards model was used to select variables to construct a nomogram. Discrimination and calibration of the nomogram were performed via the concordance index (C-index) and calibration plots. The performance of the nomogram was estimated using the calibration curve. Receiver operating characteristic (ROC) curve analysis and decision curve analysis (DCA) were performed to evaluate the predictive accuracy and net benefit of the nomogram, respectively.
Of these 142 GBC patients, 55 (38.7%) were male, and the median and mean age were 64 and 63.9 years, respectively. Forty-eight (33.8%) patients in this cohort were censored in the survival analysis. The median survival time was 20 months. A series of methods, including the likelihood ratio test and Akaike information criterion (AIC) as well as stepwise, forward, and backward analyses, were used to select the model, and all yielded identical results. Jaundice [hazard ratio (HR) = 2.9; 95% confidence interval (CI): 1.60-5.27], cCA19-9 (HR = 3.2; 95%CI: 1.91-5.39), stage (HR = 1.89; 95%CI: 1.16-3.09), and resection (R) (HR = 2.82; 95%CI: 1.54-5.16) were selected as significant predictors and combined into a survival time predictive nomogram (C-index = 0.803; 95%CI: 0.766-0.839). High prediction accuracy (adjusted C-index = 0.797) was further verified via bootstrap validation. The calibration plot demonstrated good performance of the nomogram. ROC curve analysis revealed a high sensitivity and specificity. A high net benefit was proven by DCA.
A nomogram has been constructed to predict the overall survival of GBC patients who underwent radical surgery from a clinical database of GBC at PUMCH.
Core tip: A nomogram including jaundice, carbohydrate antigen 19-9 (CA19-9), American Joint Committee on Cancer tumor node metastasis stage, and incisional margin status was built to predict the survival of gallbladder cancer patients who underwent curative resection at Peking Union Medical College Hospital. After calibration and verification, this model was shown to have high predictive accuracy and good performance.
- Citation: Bai Y, Liu ZS, Xiong JP, Xu WY, Lin JZ, Long JY, Miao F, Huang HC, Wan XS, Zhao HT. Nomogram to predict overall survival after gallbladder cancer resection in China. World J Gastroenterol 2018; 24(45): 5167-5178
- URL: https://www.wjgnet.com/1007-9327/full/v24/i45/5167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i45.5167
Gallbladder cancer (GBC) is a common biliary tract malignancy that ranks as the sixth most common digestive tract cancer[1,2]. Because of the lack of specific early screening methods and typical symptoms, most patients with GBC present with advanced-stage disease. Surgical resection remains the primary treatment for GBC because of the low sensitivity of GBC to radiotherapy (RT) and chemotherapy and because of a lack of effective drugs. Although the prevalence of GBC is low, the 5-year overall survival rate decreased from 20.1% from 2003-2005 to 16.4% from 2012-2015[3].
Although the American Joint Committee on Cancer (AJCC) staging system has published an updated eighth edition, this system does not offer precise prognostic information for individual patients[4]. Both physicians and patients are paying more attention to prognostic outcomes for GBC after surgical therapeutic interventions. Hence, a nomogram that accurately and specifically predicts overall survival is urgently needed. As a statistical predictive model, nomograms have been rapidly developed for most carcinoma types and are popular among doctors and patients because of their friendly and feasible interface[5,6]. More common tumors of the hepatobiliary system, such as hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC), have more explicit pathogenic factors and affect a relatively larger number of patients compared with GBC. Many nomograms suitable for these tumor types have been established to help clinicians accurately make rational decisions regarding diagnosis, treatment, and prognosis[7-9].
The Surveillance, Epidemiology, and End Results (SEER) Medicare database represents the American population and is an ideal research source for estimating cancer incidence and constructing survival models. In 2008, Wang et al[10] designed an individual predictive model considering the contribution of adjuvant RT to evaluate survival improvement in GBC patients after resection. However, not everyone was sensitive to RT and chemotherapy due to differences in lymph node status and distant metastasis. Therefore, in 2011, they proposed another nomogram to further clarify specific GBC populations with the potential to obtain longer survival times after adjuvant chemoradiotherapy (CRT)[11]. For chronic cholecystitis, Zhou et al[12] developed an individualized diagnostic nomogram for stage I-II GBC in chronic cholecystitis patients with gallbladder wall thickening in 2016. Recently, a more accurate and effective survival model for predicting the prognosis of patients with nonmetastatic GBC after surgical resection derived from the SEER database was built by Zhang et al[13]. However, due to the limited number of GBC patients and disparate risk factors in China, to the best of our knowledge, no predictive model has thus far been established to evaluate the prognosis of patients with GBC in China.
The current study aimed to incorporate individual correlation determinants into a nomogram to predict overall survival for GBC patients after radical resection in China.
From January 1, 2003 to January 1, 2018, 142 patients diagnosed with GBC via pathological examination after curative intent surgical resection at Peking Union Medical College Hospital (PUMCH) of the Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC) in Beijing, China were included in the current study. The inclusion criteria were as follows: (1) radical surgery; (2) GBC confirmed by pathological examination; (3) no antitumor treatment before or during surgery; (4) no other malignant tumors; and (5) pathological examination revealing a clear number of positive lymph nodes and the total number of lymph nodes obtained from the dissection. The exclusion criteria were as follows: (1) lack of a clear pathological diagnosis; (2) distant metastasis; (3) incomplete lymph node data; (4) nonprimary tumor; or (5) incomplete follow-up data.
Preoperative staging and surgical evaluation were performed based on imaging and laboratory examinations. Staging was further evaluated during surgery based on the findings and on the cryosection biopsy report. The following surgeries were performed according to the stage: for stage Tis-T1a patients, cholecystectomy was considered radical resection; for patients with stage T1b-T3/N0-1, cholecystectomy, hepatic wedge resection, and regional lymph node dissection were performed; for partial stage T3N2 patients, cholecystectomy, hepatic wedge resection, and enlarged lymph node dissection were performed; and for some patients with stage T4/N1-2, extended radical resection including combined semihepatic resection, peripheral organ resection, and hepatic pancreaticoduodenectomy were performed according to standard radical surgical procedures.
The study was approved by the Medical Ethics Committee of PUMCH of the CAMS & PUMC. All patients provided written informed consent. The study was carried out according to the ethical standards of the World Medical Association Declaration of Helsinki[14].
Demographic and clinical information and related variables were manually reviewed from the medical records. We retrospectively reviewed the medical records of patients to collect demographic data, body mass index (BMI), physical examination findings, serum laboratory test results, surgical records, pathological reports and imaging findings of cholecystolithiasis determined via ultrasonography, computerized tomography, and magnetic resonance imaging. Subjects involved in this study were those who underwent radical surgery without R2 excision and were diagnosed with GBC by histopathology. GBC stage and postoperative pathologic tumor node metastasis (pTNM) information were determined using the AJCC 8th edition (AJCC-8) classification system[4]. Incisional margins and tumor size were ascertained based on surgeon observations and final pathological assessments. All patients were followed routinely after discharge. The last follow-up time and vital status were recorded. After screening, 142 patients with confirmed GBC met the inclusion criteria.
Descriptive statistics for time-to-event variables and predictors were performed for quick screening of the data. Categorical variables are presented as numbers and percentages, and continuous variables are presented as the minimum, median, mean, maximum, and standard deviation. Some continuous variables were converted to categorical variables because their significance and linear relationships to outcomes were not satisfied after graphical and statistical assessments. For some categorical predictors, small categories were merged with others. The Kaplan-Meier (K-M) method was applied to compare survival curves for categories of individual predictors, and the log-rank test was used to determine the significance of these differences. Model selection methods, including the likelihood ratio test, Akaike information criterion (AIC), and stepwise, forward, and backward analyses, were used to construct a Cox proportional hazards model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated. Possible confounders and interactions in the model were detected. Schoenfeld residuals vs ranked survival time for selected predictors were analyzed to evaluate the proportional hazard assumption of the model. The predictive accuracy of the model was estimated by the concordance index (C-index). The overfit and predictive performance of the model were assessed via bootstrap validation. The clinically significant variables calculated from the Cox proportional hazards model were integrated into a nomogram to predict the overall survival of patients undergoing GBC resection. The performance of the nomogram was estimated using a calibration curve. The predictive accuracy and net benefit of the nomogram were assessed via receiver operating characteristic (ROC) curve analysis and decision curve analysis (DCA), respectively. The significance level for all statistical tests was set at 0.05, and all tests were two-sided. Statistical analyses were performed using R version 3.5.0 software (http://www.r-project.org/). Extension packages, including “survival”, “rms”, “nomogramEx”, and “survminer” were also used.
The study cohort consisted of 142 eligible patients who underwent GBC resection. Forty-eight (33.8%) patients were censored. The median survival time was 20 mo. The one- and 3-year survival probabilities were 63.8% and 36%, respectively (Table 1).
| Total | Event n (%) | Censored n (%) | Time (mo) | Survival probability | 95%CI | Quartile | Point estimate | 95%CI |
| 142 | 94 (66.2) | 48 (33.8) | 12 | 0.638 | 0.562-0.724 | 50% | 20 | 14-31 |
| 36 | 0.360 | 0.284-0.458 |
A detailed description of all the clinicopathologic and treatment characteristics can be found in Table 2. Notably, carbohydrate antigen 19-9 (CA19-9) as a candidate predictor spanned a wide range from a minimum value of 0.01 kU/L to a maximum value of 10524 kU/L. In addition, a large difference between the median and mean values resulted in obvious skewness. Both indicated that using CA19-9 as a continuous variable was not suitable for model construction due to its limited predictive role, as demonstrated by the relatively small coefficient. Hence, we converted CA19-9 into a categorical variable based on normal reference ranges to investigate its correlation with outcome. According to the latest AJCC staging system, we divided the patients into eight groups including stage 0, I, IIA, IIB, IIIA, IIIB, IVA, and IVB for predictor selection. We then combined stages 0 to IIIA into one category, while the remaining categories were combined due to few cases in some specific groups. In addition, pathological grade X (GX), which refers to a degree of pathological differentiation that cannot be assessed, accounted for 8.5% (12/142) of all participants and was treated as a missing value.
| Feature (Min, Median, Mean, Max, SD) | No. of patients |
| Age (35, 64, 63.9, 83, 10.2), years | |
| < 55 | 24 (16.9) |
| 55-65 | 49 (34.5) |
| ≥ 65 | 69 (48.6) |
| Gender | |
| Male | 55 (38.7) |
| Female | 87 (61.3) |
| Jaundice | |
| Absent | 122 (85.9) |
| Present | 20 (14.1) |
| Cholecystolithiasis | |
| Absent | 67 (47.2) |
| Present | 75 (52.8) |
| Diabetes | |
| Absent | 32 (22.5) |
| Present | 110 (77.5) |
| BMI (15.4, 23.5, 24.2, 32.3, 3.4) | |
| < 24 | 75 (52.8) |
| ≥ 24 | 67 (47.2) |
| CA19-9 (0.01, 13.2, 66.4, 10524, 507.7), kU/L | |
| < 40 | 65 (45.8) |
| ≥ 40 | 77 (54.2) |
| Tumor size (0.2, 3.0, 3.4, 13, 2.1), cm | |
| < 2 | 38 (26.8) |
| 2-5 | 68 (47.9) |
| ≥ 5 | 36 (25.3) |
| Primary tumor | |
| Tis | 9 (6.3) |
| T1 | 9 (6.3) |
| T2 | 20 (14.1) |
| T3 | 93 (65.5) |
| T4 | 11 (7.8) |
| Regional lymph node | |
| N0 | 86 (60.6) |
| N1 | 43 (30.3) |
| N2 | 13 (9.1) |
| Stage | |
| 0 | 9 (6.3) |
| I | 9 (6.3) |
| IIA | 10 (7.0) |
| IIB | 3 (2.1) |
| IIIA | 49 (34.6) |
| IIIB | 40 (28.2) |
| IVA | 9 (6.3) |
| IVB | 13 (9.2) |
| Histologic grade | |
| G1 | 27 (19.0) |
| G2 | 53 (37.3) |
| G3 | 50 (35.2) |
| GX | 12 (8.5) |
| Surgical margins | |
| R0 | 112 (78.9) |
| R1 | 30 (21.1) |
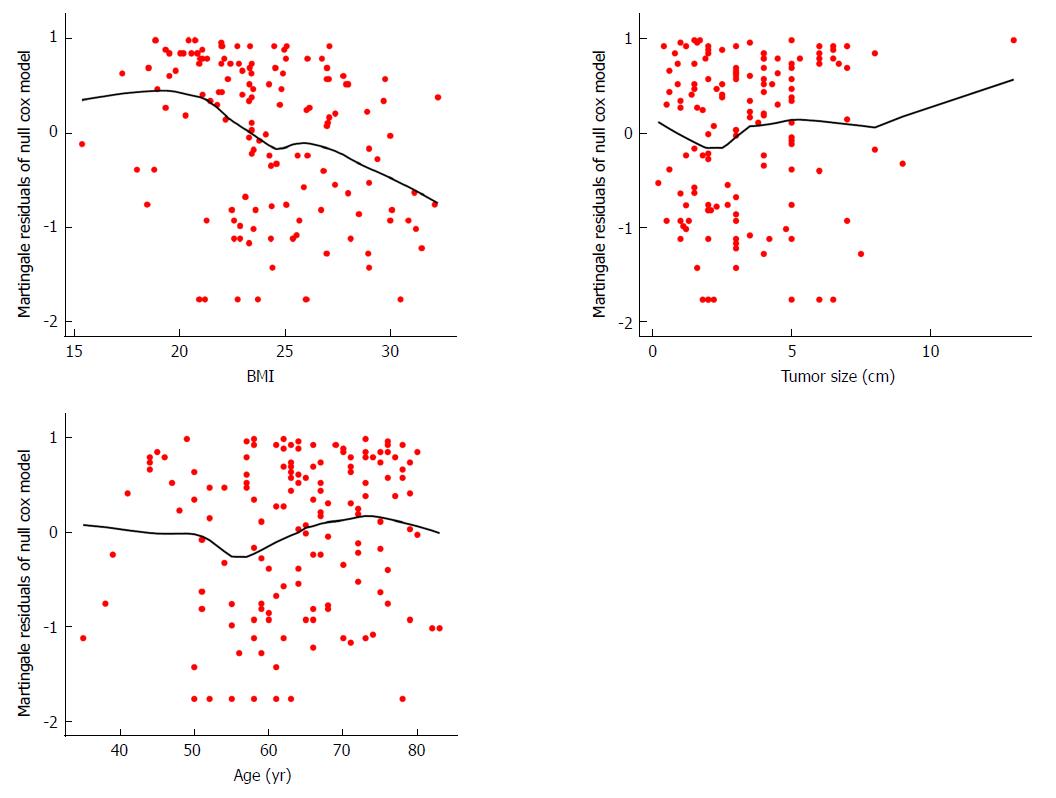
To choose the significant predictive variables that correlated well with outcome, age at diagnosis, gender, jaundice, BMI, gallstones, diabetes, tumor size, CA19-9 levels, AJCC-8 stage, tumor differentiation, and surgical margins were incorporated statistics. For continuous variables, null model residuals (martingale residuals) vs age, BMI, and tumor size plotted with LOESS lines were performed to obtain preliminary assessments of their predictive potential for survival time. As shown in Figure 1, BMI and tumor size appeared to have a considerable nonlinear relationship with martingale residuals, indicating that the linear assumption of the model for BMI and tumor size with survival time may be rejected. Log transformation was subsequently attempted for BMI and tumor size; however, the linear correlation was little improved. Continuous predictors were thus converted into categorical variables. Notably, Figure 1 illustrates that the significance cutoff for BMI was approximately 24, which was consistent with the standard value for distinguishing normal and overweight in China; therefore, we converted BMI into two categories based on this cutoff. In addition, martingale residuals for BMI were closer to the fitted line than the other two variables, suggesting that BMI may be a potential predictor. We observed a scatter located in the top right of the tumor size graph (Figure 1), which can be considered a potential outlier because it robustly influenced the tendency of the fitted line. Cutoffs of approximately 2 and 5 were a better choice, consistent with the common classification criterion. For the predictor age, we initially used a univariate Cox model to assess the correlation between age and outcome, and the results showed that it was not a significant predictor. Because the fitted line appeared to be linear, and because there was no obvious cutoff, we evenly separated age into three categories (less than 55, 55 to 65, and older than 65 years) for further study (Figure 1). After conversion to categorical variables, we defined cCA19-9, cBMI, cTumor size, and cAge as the categorical forms of these variables to distinguish them from the continuous forms. Regarding the degree of tumor differentiation, 12 samples that could not be evaluated were treated as missing values, thus resulting in only 130 observations.
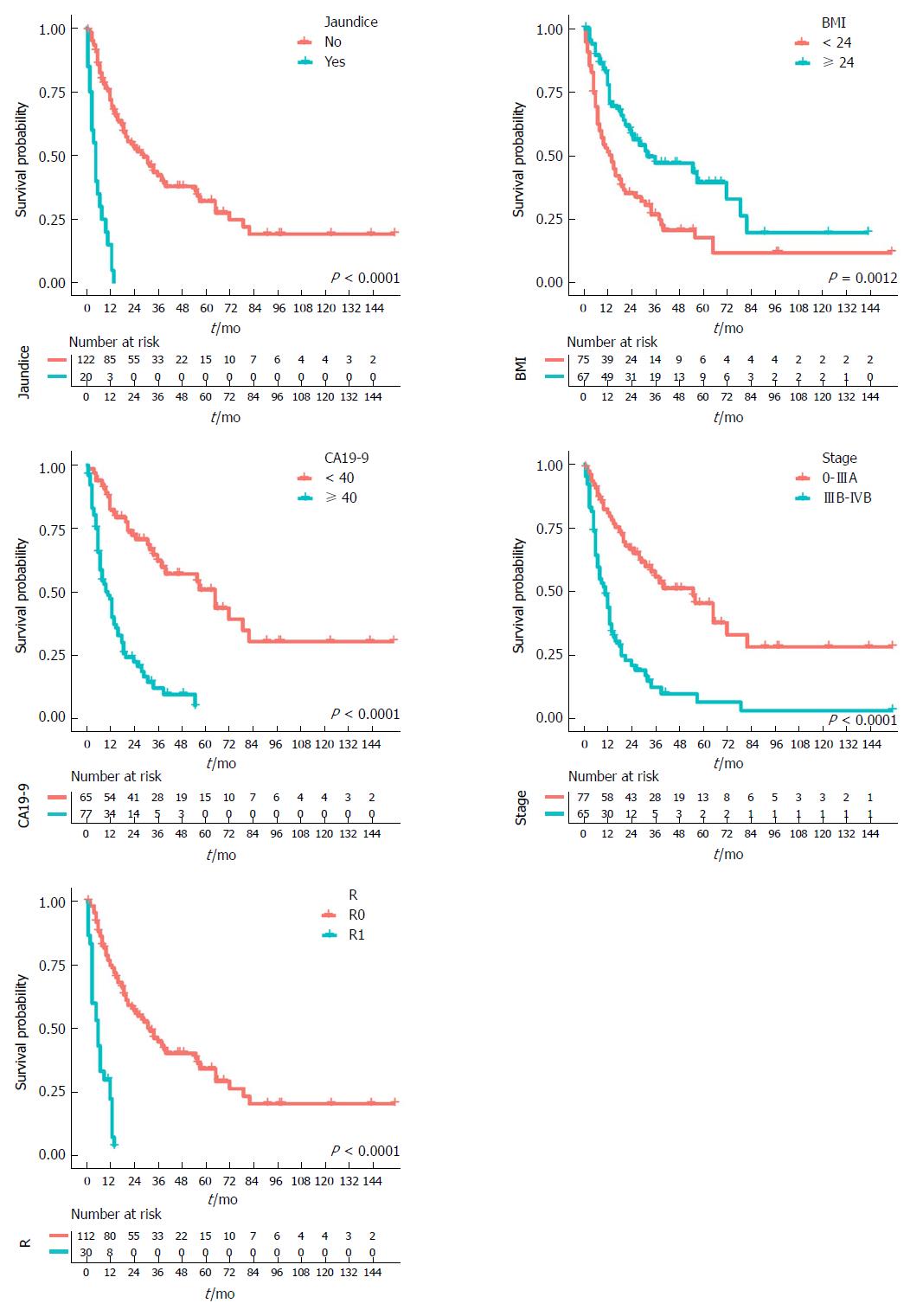
K-M survival curves for all predictors before adjustment for the other predictors were established. As shown in Figure 2, patients with jaundice had shorter survival times than patients without; patients with higher BMI exhibited longer survival times than patients with lower BMI; patients with lower CA19-9 levels showed longer survival times than patients with higher CA19-9; and patients in the lower stage group had longer survival times than patients in the higher stage group. Considering the surgical margin status, patients in the R0 category had longer survival times than patients in the R1 category. Risk tables for each indicator are shown below the corresponding K-M curves. Note that all categories of the following predictors had significant differences after the log-rank test: jaundice, cBMI, cCA19-9, stage, and R. However, cAge, gender, cholecystolithiasis, diabetes, cTumor size, and grade failed to reach significance in constructing the model.
Because the aforementioned predictors contained no missing values after excluding the degree of tumor differentiation, various model-selection criteria, including the likelihood ratio test, AIC, and stepwise, forward, and backward analyses, were utilized to construct the model for all 142 observation points. Notably, all methods yielded identical results.
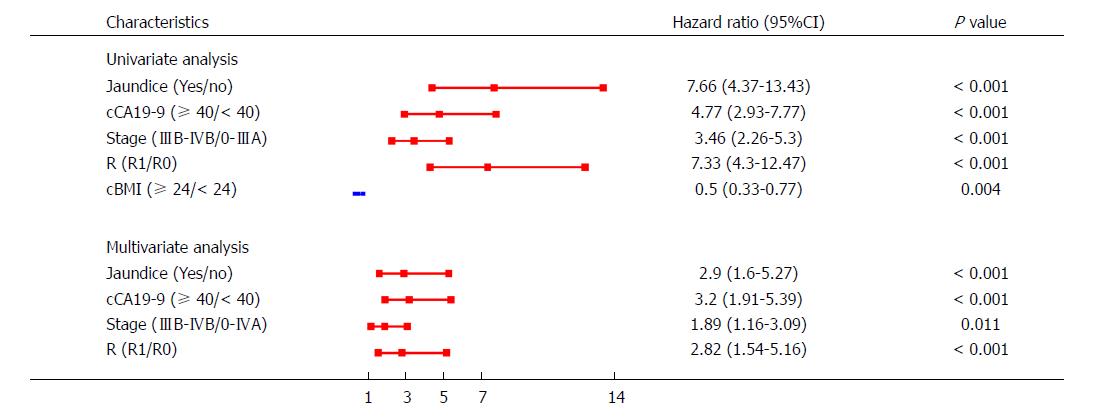
To check for possible confounders, both univariate and multivariate models were established. Compared with the univariable model, the multivariable model showed that the CIs for jaundice (95%CI: 1.60-5.27), cCA19-9 (95%CI: 1.91-5.39), stage (95%CI: 1.16-3.09), and R (95%CI: 1.54-5.16) did not change significantly after combination with other predictors (Figure 3). Furthermore, we found that cCA19-9 was a confounder for BMI levels; thus, cBMI was excluded in the multivariate model. Moreover, interactions between each pair of predictors were examined, and no interactions were detected.
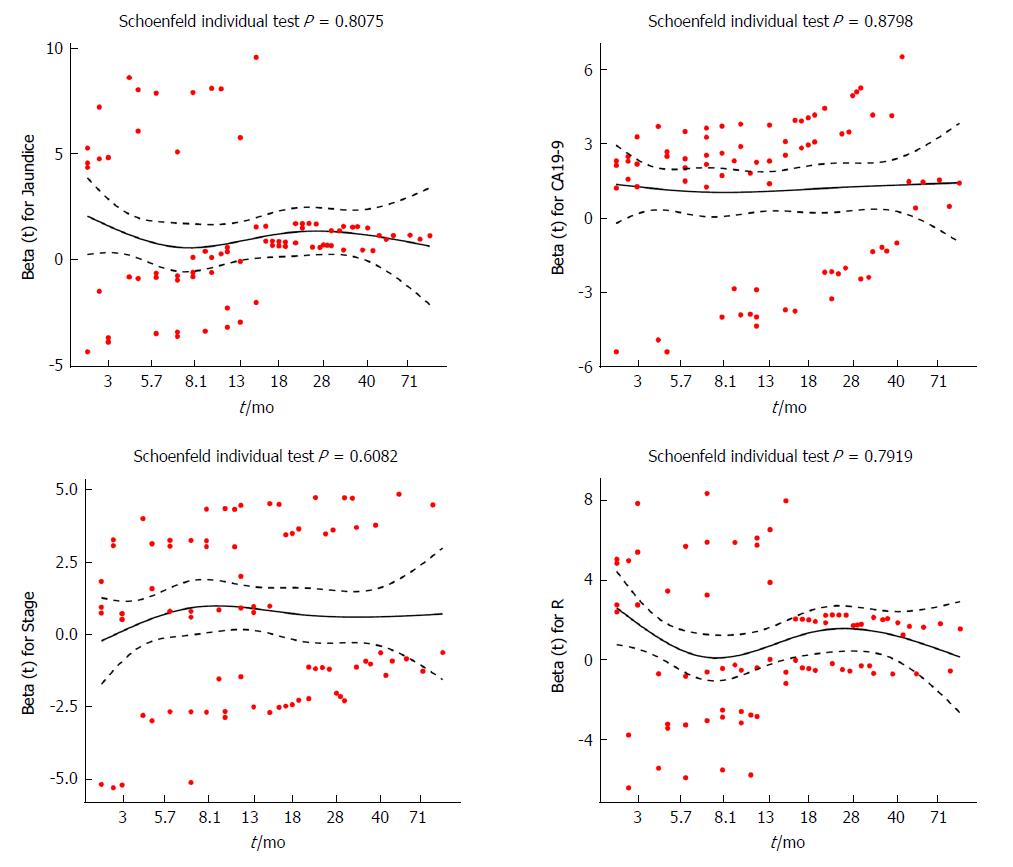
To further evaluate whether the proportional hazards assumption was valid, Schoenfeld residuals were analyzed with respect to ranked survival time for selected predictors. All fitted lines derived from individual scatter plots seemed to be horizontal (Figure 4). Furthermore, statistical tests were performed on Schoenfeld residuals vs ranked survival time for each predictor. The P-values for jaundice, cCA19-9, stage, and R were 0.8075, 0.8798, 0.6082, and 0.7919, respectively. The P-value for the global test was 0.9837. In conclusion, all the results indicated that the proportional hazards assumption was satisfied.
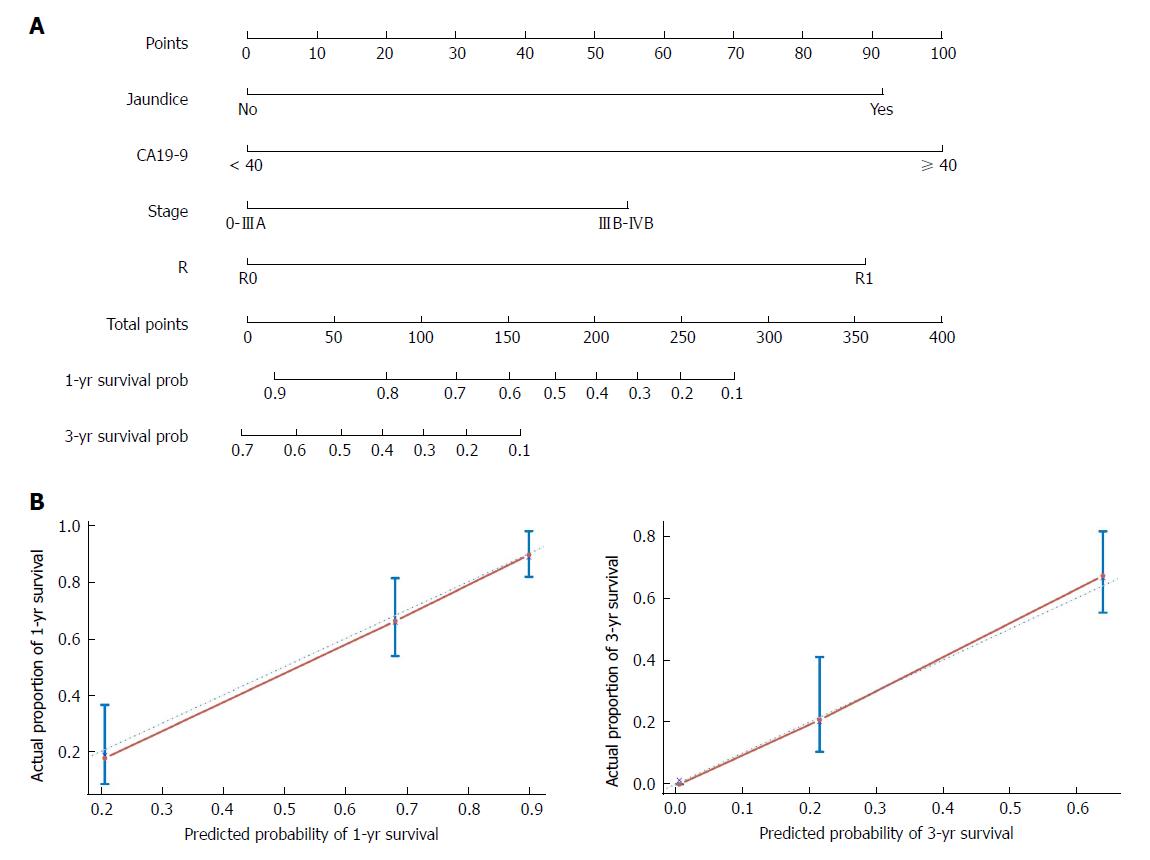
The predictive ability of the model was assessed by calculating the C-index, which was 0.803 (95%CI: 0.766-0.839). Bootstrap validation was applied to estimate the overfit of the model. The adjusted C-index representing the bias-corrected estimate of model performance in the future was 0.797 after 1000 iterations, demonstrating good predictive accuracy for the nomogram.
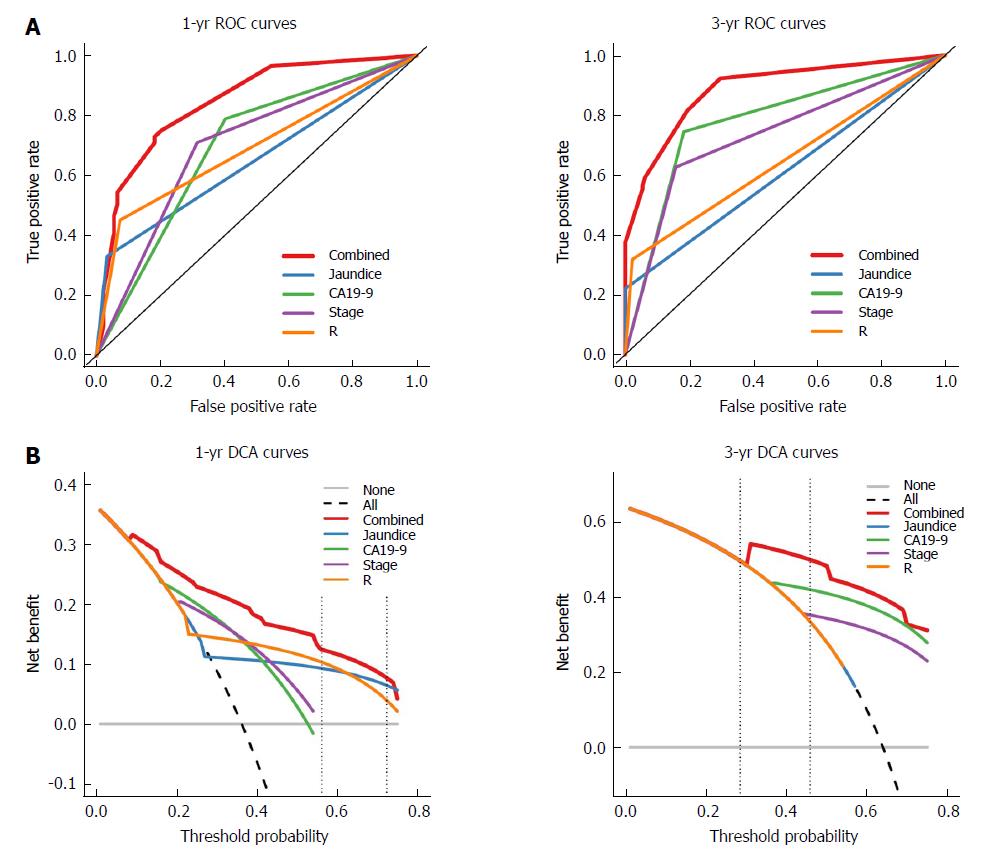
The nomogram that predicts the survival time of patients with GBC after surgical resection is displayed in Figure 5A. The nomogram was developed based on the results of the Cox proportional hazards model in Figure 3. In this nomogram, each factor was ascribed a weighted point total that indicated a survival prognosis. One- and three-year survival probabilities can be measured using this nomogram. For instance, the presence of jaundice was assigned 92 points, while a CA19-9 level ≥ 40 kU/L was assigned 100 points. The higher a patient scores, the poorer the prognosis. In addition, the performance of the nomogram was graphically evaluated using a calibration curve (Figure 5B). The predicted line overlapped well with the reference line, demonstrating the good performance of the nomogram. Similarly, we compared the predictive accuracy between the combined model and individual predictors, including jaundice, CA19-9, stage, and R, via ROC curve analysis. The area under the curve (AUC) of the nomogram was significantly larger than those of other single variables (Figure 6A). Finally, to determine whether the predictive nomogram was clinically useful, DCA was performed to evaluate the net benefit of the models. Compared with jaundice, CA19-9, stage, and R, the combined model offered the best clinical utility, as calculated within the favorable probability. Hence, this nomogram is the best model for predicting GBC patient survival, which might help clinicians with patient counseling, decision-making, and follow-up scheduling.
GBC is a common biliary tract tumor around the world. Due to its occult onset and lack of specific symptoms and early screening methods, most GBC patients already present with advanced-stage disease at diagnosis, which results in difficulty implementing curative intent surgical resection. GBC always behaves as a highly malignant tumor with a dismal prognosis[15,16]. Less than 5% of GBC patients survive for longer than 5 years[1]. The five-year survival rate of GBC patients has been declining in China according to the latest statistical report[3]. Obtaining accurate prognostic information is necessary to help physicians make better clinical decisions and perform consultations with patients regarding life expectancy after resection of tumor masses. Nomograms are alternative prognostic assessment tools for most cancers because they include more clinically related factors and offer more reliable prognostic information tailored to individual patients than the traditional AJCC TNM staging system. Nomograms are predictive tools that generate user-friendly graphical interfaces to calculate probabilities of clinical outcomes, such as diagnosis, recurrence, and prognosis, based on related, statistically significant variables[5,6,17]. The present study was the first to propose a nomogram for predicting the survival times of patients undergoing GBC resection in China. The nomogram suggested that the absence of jaundice, lower preoperative CA19-9 levels, lower AJCC TNM stage, and incisional margins without tumor cells correlated well with a long survival time. Notably, given the very broad data distribution and considerable discrepancies between the median and mean, we converted CA19-9 into a categorical variable to evaluate its relationship with outcome. In addition, we demonstrated that categorical forms of continuous variables, including BMI, tumor size, and age, were better choices for model selection. Moreover, for 12 patients with GX disease who were treated as having missing values, we did not use conventional modeling methods to first construct and then validate a nomogram. Instead, we first checked the predictive potential of each candidate variable. Importantly, after statistical analysis, we found that cCA19-9 was a confounder for BMI levels and thus excluded cBMI from nomogram construction.
The SEER database of the National Cancer Institute, which represents approximately 26% of the US population, can be used to obtain enough clinical information on rare tumor types, such as GBC. Wang et al[10,11] successively built two nomograms derived from the SEER database to evaluate the survival benefit of adjuvant RT, adjuvant chemotherapy, or CRT for patients with GBC. The first model demonstrated that patients with node-positive and/or T2 stage or higher disease had the greatest benefit from adjuvant RT[10]. The second nomogram found that patients with at least T2 or N1 disease had a survival benefit from adjuvant CRT[11]. Both studies indicated the potential for age at diagnosis, gender, race, extent of the primary tumor, and nodal status to influence the survival time of GBC patients. Interestingly, there are some differences between our results. The main reason may be that the study patients were from two different countries, leading to heterogeneity in ethnicity. In addition, environmental factors, such as living conditions, eating habits, and other risk factors, may also have contributed to the different results[16,18,19]. Furthermore, GBC is a rare tumor in China; thus, large, multi-institutional study cohorts are lacking. In addition, we lacked a population-based cancer registry database similar to the SEER database. Our cohort was thus relatively small, and it was difficult to perform the same study strategy, which caused discrepancies in the results.
Recently, Zhang et al[13] constructed a model to predict the survival of patients with nonmetastatic GBC after surgical resection derived from the SEER database. Compared with the studies by Wang et al[10,11], they identified additional predictors, including tumor size, histological grade, lymph node excision, and chemotherapy. Their nomogram performed better than the seventh edition of the AJCC Cancer Staging system, further demonstrating the superiority of nomograms. Among these variables, tumor size, which had no correlation with prognosis in our model, was treated as two classified variables in their model. The AJCC standard does not use tumor size to assess T stage, which is in line with our results, indicating that tumor size plays a minor role in survival in GBC. Notably, continuous variables do not typically have a purely linear relationship with prognosis. After analyzing martingale residuals vs BMI, tumor size, and age, we found that converting these continuous variables into categorical variables was a better strategy for model selection. Furthermore, in contrast to conventional modeling methods, due to missing data regarding the grade variable, we first evaluated potential factors one by one to select five variables, including jaundice, cBMI, cCA19-9 levels, stage, and R that significantly affected outcome. For cCA19-9, which was a confounder of BMI levels and showed no interactions with each pair of predictors, four predictors other than cBMI were considered to establish the final nomogram. Overall, our research strategy was particularly suitable for a small study sample and single-center rare tumor cohorts, especially those with partial missing data. More importantly, our nomogram, which was based on a previously reported strategy, exhibited high predictive accuracy (C-index: 0.803; 95%CI: 0.766-0.839) and model performance (adjusted C-index: 0.797).
There are several potential limitations in this study. Our research cohort was from a single institution (PUMCH, which is one of the most famous hospitals where GBC patients from Beijing and the surrounding can seek diagnosis and treatment) with a small clinical database. The study results may not be widely used in patients from other institutions or countries because of selection bias and the lack of external validation. However, compared with patient cohorts from some other institutions in China, the clinical characteristics were similar, indicating the individualized epidemiology of GBC in China[20]. In addition, these shortcomings may to some extent be transformed into advantages because compared with large-scale multicenter studies, a predictive nomogram built from a single institution study may have a high sensitivity and specificity due to decreased heterogeneity caused by demographic, clinical, and tumor-related characteristics. Clinicians are devoted to constructing models with general applicability and high accuracy at all times. However, this aim is always hard to fulfill due to the contradictions between heterogeneity and homogeneity. The establishment of a nomogram based on a single institution for survival prediction of rare tumors may be an alternative choice. Here, we introduced the details of a modeling method to facilitate the wide application of this research strategy.
In summary, jaundice, preoperative CA19-9 levels, AJCC-8 stage, and surgical margin status played vital roles in influencing survival time and were incorporated into a nomogram to predict outcomes for postoperative GBC patients. This model had high predictive accuracy and performed well after bootstrap validation and calibration. This type of research strategy should be widely used to construct specific nomograms according to different institutional databases, especially for rare tumors with small patient sample sizes with some missing data.
Gallbladder cancer (GBC) is a rare tumor type with dismal outcomes. With advances in medical science, GBC patients have more treatment choices in addition to surgical resection, including chemotherapy, radiotherapy, targeted therapy, and immunotherapy. However, 5-year survival rates are surprisingly decreasing in China. Hence, screening GBC prognostic risk factors and constructing a prognostic model with high predictive accuracy and clinical utility for assessing the survival time of patients undergoing curative intent resection for GBC are of great importance.
Nomograms can integrate several independent prognostic factors for tumor patients into one model according by weighting each indicator to predict their overall survival. Compared with a single prediction indicator, this method can therefore provide more accurate and personalized prognostic information. Unfortunately, because of rare samples and ambiguous risk factors, nomograms to estimate survival time in GBC patients, especially in China, remain limited.
To establish a nomogram with easy use and high performance for predicting the survival of GBC patients undergoing radical resection in China, which will help doctors make rational decisions with respect to treatment, prognosis, and follow-up.
To select survival-related predictors, clinical parameters consisting of age, gender, jaundice, cholecystolithiasis, diabetes, body mass index (BMI), carbohydrate antigen 19-9 (CA19-9), tumor size, pathological stage, histologic grade, and surgical margins derived from 142 GBC patients after curative intent surgical resection at Peking Union Medical College Hospital (PUMCH) were incorporated into a univariate Cox regression analysis. Model selection criteria, including the likelihood ratio test, Akaike information criterion (AIC), and stepwise, forward, and backward analyses, were applied. Jaundice, CA19-9, pathological stage, and resection (R) were combined into a survival-time predictive nomogram. The predictive accuracy of the model was estimated using the concordance index (C-index). The performance of the nomogram was estimated using a calibration curve. The predictive accuracy and net benefit of the nomogram were assessed via receiver operating characteristic (ROC) curve analysis and decision curve analysis (DCA), respectively.
A nomogram consisting of jaundice, CA19-9 levels, pathological stage, and resection margin status was constructed to predict the survival time of GBC patients after curative resection. More importantly, our nomogram exhibited high predictive accuracy (C-index: 0.803; 95%CI: 0.766-0.839) and model performance (adjusted C-index: 0.797). Due to limited samples, more samples are needed to optimize model performance.
A nomogram was constructed to predict the overall survival of GBC patients who underwent radical surgery from a clinical database of GBC at PUMCH. In addition to a conventional nomogram construction strategy, continuous predictors were first converted into categorical variables after graphical assessment. Then, optimal cutoffs were selected regarding both normal references and martingale residuals. Schoenfeld residuals were analyzed with respect to ranked survival time for selected predictors, including jaundice, CA19-9 levels, pathological stage, and R, to further evaluate whether the proportional hazards assumption was valid. Finally, the predictive accuracy and clinical utility of nomogram were checked via ROC curve analysis and DCA, respectively. In summary, this study not only introduced a novel nomogram construction method to optimize model performance but also provided more detail information for clinicians to perform patient counseling, decision-making, and follow-up scheduling.
This study describes a modeling method based on a single institution for survival prediction of rare tumors. This model had high predictive accuracy and performed well after bootstrap validation and calibration. This research strategy should be widely used to construct specific nomograms according to different institutional databases, especially for rare tumors with small sample sizes of patients with some missing data.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Higuchi K, Jung DH, Seo DW S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 420] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 2. | Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am. 2014;94:343-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 795] [Cited by in F6Publishing: 773] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 4. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2341] [Cited by in F6Publishing: 3379] [Article Influence: 482.7] [Reference Citation Analysis (2)] |
| 5. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1119] [Cited by in F6Publishing: 1952] [Article Influence: 216.9] [Reference Citation Analysis (0)] |
| 6. | Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1306] [Cited by in F6Publishing: 2023] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 7. | Groot Koerkamp B, Wiggers JK, Gonen M, Doussot A, Allen PJ, Besselink MG, Blumgart LH, Busch OR, D'Angelica MI, DeMatteo RP. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2015;26:1930-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Hyder O, Marques H, Pulitano C, Marsh JW, Alexandrescu S, Bauer TW, Gamblin TC, Sotiropoulos GC, Paul A, Barroso E. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014;149:432-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Wan G, Gao F, Chen J, Li Y, Geng M, Sun L, Liu Y, Liu H, Yang X, Wang R. Nomogram prediction of individual prognosis of patients with hepatocellular carcinoma. BMC Cancer. 2017;17:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Wang SJ, Fuller CD, Kim JS, Sittig DF, Thomas CR Jr, Ravdin PM. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112-2117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Wang SJ, Lemieux A, Kalpathy-Cramer J, Ord CB, Walker GV, Fuller CD, Kim JS, Thomas CR Jr. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol. 2011;29:4627-4632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Zhou D, Wang JD, Yang Y, Yu WL, Zhang YJ, Quan ZW. Individualized nomogram improves diagnostic accuracy of stage I-II gallbladder cancer in chronic cholecystitis patients with gallbladder wall thickening. Hepatobiliary Pancreat Dis Int. 2016;15:180-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Zhang W, Hong HJ, Chen YL. Establishment of a Gallbladder Cancer-Specific Survival Model to Predict Prognosis in Non-metastatic Gallbladder Cancer Patients After Surgical Resection. Dig Dis Sci. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14-18. [PubMed] [Cited in This Article: ] |
| 15. | Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, Coimbra FJ, Jarnagin WR. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015;17:681-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 16. | Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 528] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 790] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 18. | Figueiredo JC, Haiman C, Porcel J, Buxbaum J, Stram D, Tambe N, Cozen W, Wilkens L, Le Marchand L, Setiawan VW. Sex and ethnic/racial-specific risk factors for gallbladder disease. BMC Gastroenterol. 2017;17:153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55:218-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Shen HX, Song HW, Xu XJ, Jiao ZY, Ti ZY, Li ZY, Ren B, Chen C, Ma L, Zhao YL. Clinical epidemiological survey of gallbladder carcinoma in northwestern China, 2009-2013: 2379 cases in 17 centers. Chronic Dis Transl Med. 2017;3:60-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |