Published online Oct 14, 2018. doi: 10.3748/wjg.v24.i38.4341
Peer-review started: June 21, 2018
First decision: August 1, 2018
Revised: August 6, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: October 14, 2018
Processing time: 113 Days and 12.8 Hours
To investigate the temporal clinical, proteomic, histological and cellular immune profiles of dextran sulfate sodium (DSS)-induced acute colitis.
Acute colitis was induced in C57Bl/6 female mice by administration of 1%, 2% or 3% DSS in drinking water for 7 d. Animals were monitored daily for weight loss, stool consistency and blood in the stool, while spleens and colons were harvested on day 8. A time course analysis was performed in mice ingesting 3% DSS, which included colon proteomics through multiplex assay, colon histological scoring by a blinded investigator, and immune response through flow cytometry or immunohistochemistry of the spleen, mesenteric lymph node and colon.
Progressive worsening of clinical colitis was observed with increasing DSS from 1% to 3%. In mice ingesting 3% DSS, colon shortening and increase in pro-inflammatory factors starting at day 3 was observed, with increased spleen weights at day 6 and day 8. This coincided with cellular infiltration in the colon from day 2 to day 8, with progressive accumulation of macrophages F4/80+, T helper CD4+ (Th), T cytotoxic CD8+ (Tcyt) and T regulatory CD25+ (Treg) cells, and progressive changes in colonic pathology including destruction of crypts, loss of goblet cells and depletion of the epithelial barrier. Starting on day 4, mesenteric lymph node and/or spleen presented with lower levels of Treg, Th and Tcyt cells, suggesting an immune cell tropism to the gut.
These results demonstrate that the severity of experimental colitis is dependent on DSS concentration, correlated with clinical, proteomic, histological and cellular immune response on 3% DSS.
Core tip: Our study contributes to a better understanding of the dextran sulfate sodium (DSS) acute colitis model in order to provide a stronger basis for novel therapies. Colonic proteomic temporal analysis reveals an increase in cytokines with a strong influx of immune cells. The highest cytokine levels were observed when animals were no longer drinking DSS, suggesting a rebound response. Secondary lymphoid organs contribute by sending different immune cells to the colon during the acute phase, such as CD4+, CD8+ and CD25+ T cells. Our results demonstrate involvement of the adaptive and innate immune responses during the acute phase of DSS-induced colitis.
- Citation: Nunes NS, Kim S, Sundby M, Chandran P, Burks SR, Paz AH, Frank JA. Temporal clinical, proteomic, histological and cellular immune responses of dextran sulfate sodium-induced acute colitis. World J Gastroenterol 2018; 24(38): 4341-4355
- URL: https://www.wjgnet.com/1007-9327/full/v24/i38/4341.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i38.4341
Inflammatory bowel diseases (IBD) are chronic inflammatory diseases and consist mainly of ulcerative colitis (UC) and Crohn’s disease (CD). UC usually presents with symptoms of diarrhea, weight loss, abdominal pain and blood in the stool and the development of IBD is associated with genetic, environmental and microbial factors[1,2]. Despite the rapid rise of IBD in the United States (US) and Europe, even with the advent of biological therapies, there are no current treatments that will sustain remission. Numerous animal models, including the chemically inducible colitis model of dextran sulfate sodium (DSS), have been developed to understand the pathobiology of IBD and evaluate novel therapies[3-5]. When DSS is added to drinking water, mice develop colitis that can be modulated by altering the DSS concentration, molecular weight and microbiota[6,7]. DSS primarily causes disruption of the intestinal barrier, allowing access of antigens and pro-inflammatory factors from the intestinal contents to the mucosal layer of the large bowel. Moreover, the exact mechanism has not been thoroughly elucidated[6,8,9]. Histological characteristics of DSS colitis includes the depletion of crypts, infiltration of neutrophils, ulceration and inflammation of the mucosal and submucosal layers[10]. Initial studies[11] suggested that human UC was predominantly associated with a Th2 immune response [interleukin (IL)-5], however it has been shown that other factors from Th1 [tumor necrosis factor alpha (TNFα)] and Th17 (IL-17, IL-23) profiles are also implicated in the development of the disease[12-15]. Although the DSS acute and chronic colitis models are not solely dependent on B and T cell responses, a complex interplay between innate and adaptive immune system occurs, in which neutrophils (N), eosinophils (E), macrophages (M), dendritic cells (DC), T cells and B cells participate in the exaggerated presentation of the disease[15-18].
Previous studies have individually investigated the clinical manifestations of DSS induced colitis with temporal proteomic, immune cells infiltration, histological changes in the colon and transcriptional genomics[9,15,16,19-21]. In the current study, the relationship between daily clinical activities along with temporal molecular analysis, histological features and immune cell trafficking were investigated during the acute phase of DSS colitis, to further the understanding of the interaction of these factors in disease development.
The protocol was approved by the Animal Care and Use Committee at our institution. C57BL/6 female mice 6-8 wk old from Charles River Laboratories (Wilmington, MA, United States) were used for the experiments. Animals were housed in specific pathogen-free conditions with 12h-12h light-dark cycles under controlled humidity and temperature.
Experimental acute colitis was induced by administration of 1%, 2% or 3% (wt/vol) DSS (36000-50000 Da - MP Biomedicals, Solon, OH, Unites States) in drinking water ad libitum for 7 d and euthanized at day 8. Control animals were allowed sterilized tap water ad libitum. For the time course analysis, mice received 3% DSS for 7 d and were euthanized on day 0, day 2, day 3, day 4, day 5, day 6 and day 8. Euthanasia was performed through isoflurane anesthesia followed by cervical dislocation for collection of biological samples.
Animals (n = 6/DSS group and n = 6/time point) were daily evaluated through disease activity index (DAI), as previously described[7,22]. Table 1 contains the grading criteria used for the DSS colitis model. Briefly, animals were evaluated for weight loss (0-4), stool consistency (0-4) and blood in the stool (0-4), in which DAI reaches a maximum score of 12. After euthanasia at different time points, the entire colon was collected and cleaned with flushing PBS (Phosphate buffered saline) 1 ×. Colon was weighed, and the colon length was measured from the caecum to the anus. Spleen was weighted and further processed for flow cytometry analysis.
| Score | Weight loss | Stool consistency | Bleeding |
| 0 | None | Normal | No bleeding |
| 1 | 1%-5% | - | - |
| 2 | 5%-10% | Loose stools | Slight bleeding |
| 3 | 10%-15% | - | - |
| 4 | More than 15% | Watery diarrhea | Gross bleeding |
Colon samples from animals (n = 6/each time point) receiving 3% DSS were snap frozen and later homogenized for protein extraction. Briefly, frozen colon samples were processed in cell lysis buffer containing 150 mmol/L NaCl, 1 mmol/L EDTA, 20 mmol/L Tris-HCl and 0.05% Tween-20, with addition of protease inhibitor (Thermo Scientific, Waltham, MA, United States) and 1.0 mm Zyrconium Beads. Samples were centrifuged twice at 14000 r/min at 4 °C for 20 min and supernatant was collected. Aliquots were kept at -80 °C until further analysis. Samples were quantified through bicinchoninic acid assay (BCA - Thermo Scientific, Waltham, MA, United States) and diluted to a final concentration of 1 mg/mL of total protein. Colon homogenates were analyzed by MILLIPLEX Map Mouse Cytokine/Chemokine Panel (EMD Millipore, Billerica, MA, United States) using Bio-Plex 200 (Bio-Rad) according to manufacturer specifications.
Spleen and mesenteric lymph nodes (MLN) (n = 6/each time point) were collected and processed for flow cytometry analysis. Tissue samples were smashed between two frosted glass slides in the presence of ammonium-chloride-potassium lysing buffer (Lonza, Walkersville, MD, United States) until they were dissociated. PBS 1 × was added and samples were centrifuged at 1500 r/min at 4 °C for 10 min. Cells were re-suspended in PBS 1 ×, filtered through a 70 μm filter and centrifuged at 1500 r/min at 4 °C for 10 min. The pellet was incubated in 10% formalin for 35 min at 4 °C and washed in PBS 1 ×. Samples were kept at 4 °C until flow cytometry analysis. The single cell suspension was incubated with the proper amounts of antibodies in Stain Buffer (BD Pharmingen, San Jose, CA, United States) for 35 min on ice protected from light, following manufacturer instructions. Samples were loaded in a V-bottom 96-well plate and read in Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA, United States). Data were analyzed using Accuri C6 Flow Cytometer software. Immune cells were characterized for T helper cells (CD3+CD4+), T regulatory cells (CD3+CD4+CD25+), T cytotoxic cells (CD3+CD8+), B cells (B220+) and Macrophages (F4/80+). Antibodies used were FITC F4/80 (Rat, 0.5 mg/mL, eBioscience), PE CD25 (Rat, 0.2 mg/mL, BD Pharmingen), Alexa Fluor 488 B220 (Rat, 0.5 mg/mL, Biolegend), APC CD4 (Rat, 0.2 mg/mL, BD Pharmingen), FITC CD3 (Rat, 0.5 mg/mL, BD Pharmingen) and PE CD8a (Rat, 0.2 mg/mL, BD Pharmingen). Enriched F4/80+ and CD3+CD4+CD25+ populations were separated prior to flow cytometry analysis using Magnetic Cell Separation MicroBeads (MACS - Miltenyi Biotec, Bergisch Gladbach, Germany) following manufacturer instructions in CD4+CD25+ Regulatory T cell isolation kit and with F4/80 MicroBeads Ultrapure. There were collected 20000 events for each sample and results are presented as the mean ± SD percentage of the total number of cells. Isotypes were also analyzed for each antibody and sample. Flow cytometry gating can be found in Supplementary Figures 1 and 2.
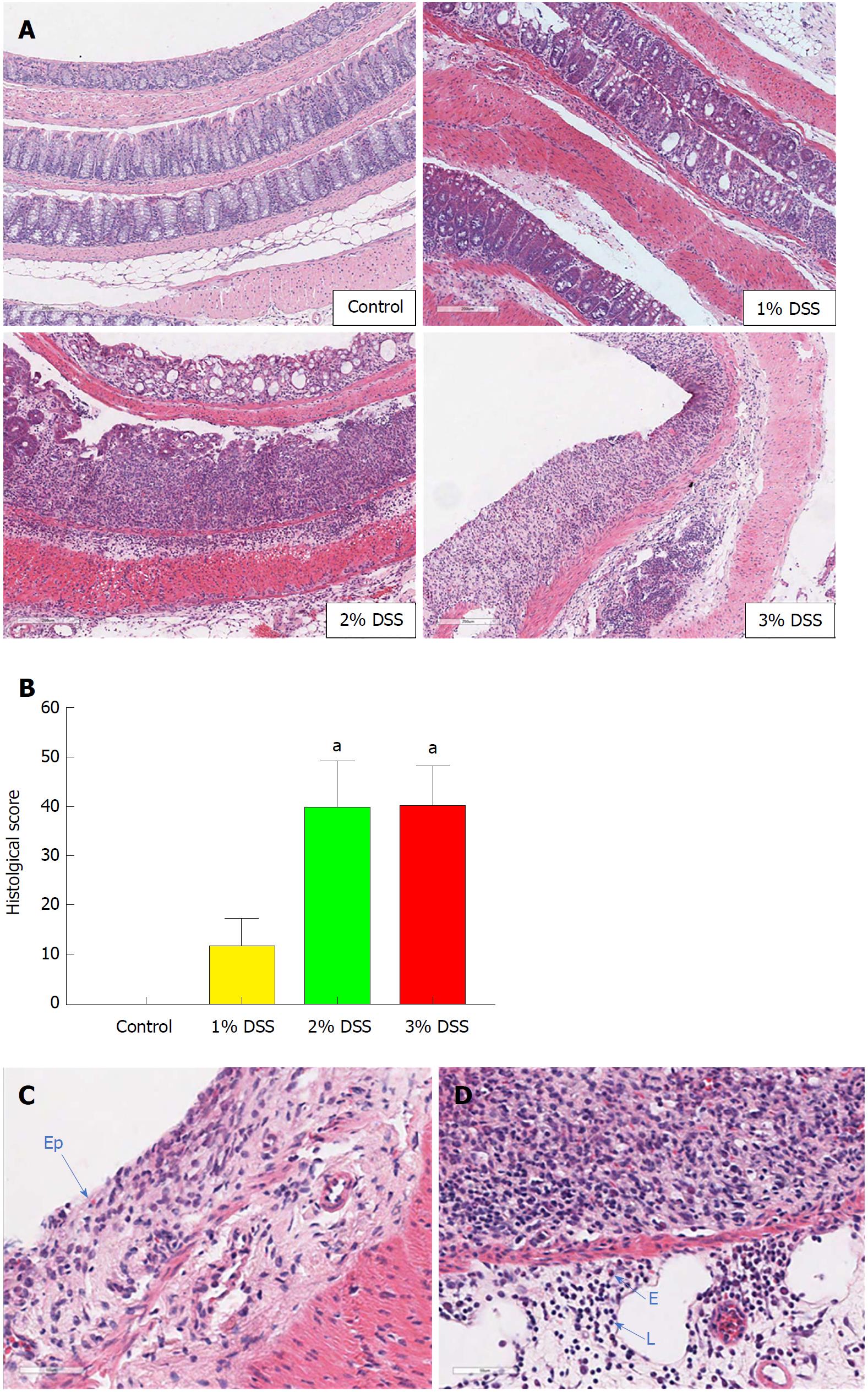
For bright field microscopy, colon samples (n = 4/DSS group and n = 4/each time point) freshly collected from animals receiving 3% DSS were washed with PBS 1 ×, cut longitudinally and kept in 10% NBF (neutral buffered formalin) as a Swiss roll for 24 h at room temperature (RT). Tissue was kept in PBS 1 × until further processed into paraffin blocks. 3 μm sections were stained with Guills II hematoxylin and Eosin-Y (HE) for morphologic analysis. The histological evaluation was performed as previously described[7]. Briefly, the tissue was analyzed for grade of inflammation (0-3), extent within the intestine layers (0-3), regeneration (0-4), crypt damage (0-4) and percentage of involvement (0-4), reaching a maximum score of 56 (Table 2)[23]. Images were obtained on a Leica Aperio ScanScope CS using a 20 × air objective (NA = 0.75, Leica Microsystems, Buffalo Grove, IL, United States) and Aperio ImageScope software. HE staining was done on spleen and MLN using the same method as colon samples.
| Feature graded | Grade | Description |
| Inflammation | 0 | None |
| 1 | Slight | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Regeneration | 4 | No tissue repair |
| 3 | Surface epithelium not intact | |
| 2 | Regeneration with crypt depletion | |
| 1 | Almost complete regeneration | |
| 0 | Complete regeneration or normal tissue | |
| Crypt damage | 0 | None |
| 1 | Basal 1/3 damaged | |
| 2 | Basal 2/3 damaged | |
| 3 | Only surface epithelium intact | |
| 4 | Entire crypt and epithelium lost | |
| Percent involvement | 1 | 1%-25% |
| 2 | 26%-50% | |
| 3 | 51%-75% | |
| 4 | 76%-100% |
For immunohistochemistry studies, FFPE (formalin fixed paraffin embedded) 3 μm colon and MLN samples (n = 4/each time point) were cut using a Leica Manual Microtome, mounted on adhesive slides, left at 20 °C overnight and then baked for 1 h at 65 °C the next day. Samples were incubated in antigen unmasking solution (citrate-based, pH = 6.0; Vector Laboratories, Burlingame, CA, United States) at 100 °C for 40 min and blocked with SuperBlock Blocking Buffer (Thermo Scientific, Waltham, MA, United States) for 20 min at RT. Primary antibodies CD4 (Rabbit, 0.623 mg/mL, Abcam), CD8 (Rabbit, 1 mg/mL, Abcam), F4/80 (Rabbit, 0.23 mg/mL, Novus Biologicals), B220 (Rat, 0.5 mg/mL, Invitrogen) and CD25 (Goat, 0.2 mg/mL, Invitrogen) were incubated at RT for 1 h. Samples were incubated with Peroxidazed 1 (BioCare Medical, Pacheco, CA, United States) for 5 min at RT, followed by incubation with the respective secondary HRP (Horseradish Peroxidase) antibody for 30 min at RT. Samples were incubated with Immpact DAB (3,3-diaminobendizine) Peroxidase HRP substrate (Vector Laboratories, Burlingame, CA, United States) for 5 min at RT. All samples were counterstained for 5 min with warmed 60 °C Methyl Green (Vector Laboratories, Burlingame, CA, United States). Respective isotypes were also analyzed. Images were obtained on a Leica Aperio ScanScope CS using a 10 × air objective (NA = 0.75, Leica Microsystems, Buffalo Grove, IL, United States) and Aperio ImageScope software. Photomicrographs were obtained from the whole area of the colon or MLN and analyzed through ImageJ. The quantification was done following the ratio of positive cells by the total area, multiplied by 100 and represented in percentage.
Statistical analysis was performed using Prism 7 (Graph Pad Inc., La Jolla, CA, United States). Experiments were evaluated through multiple student’s t-test and one-way ANOVA followed by Dunnett post-hoc test. P < 0.05 was considered statistically significant. Data are presented as mean ± SD.
This study demonstrates the progressive aggressiveness of colitis with increasing DSS concentration from 1%-3% based on clinical and histological results. That led us to focus on the evaluation of the proteomic profile and immune cell infiltration in the colon of mice ingesting 3% DSS. We observed worsening of colonic pathology with lymphocytic, macrophage and eosinophilic infiltration that was associated with increasing pro and anti-inflammatory cytokines, chemokines and trophic factors (CCTF) expression in the colon over day 2 to day 8.
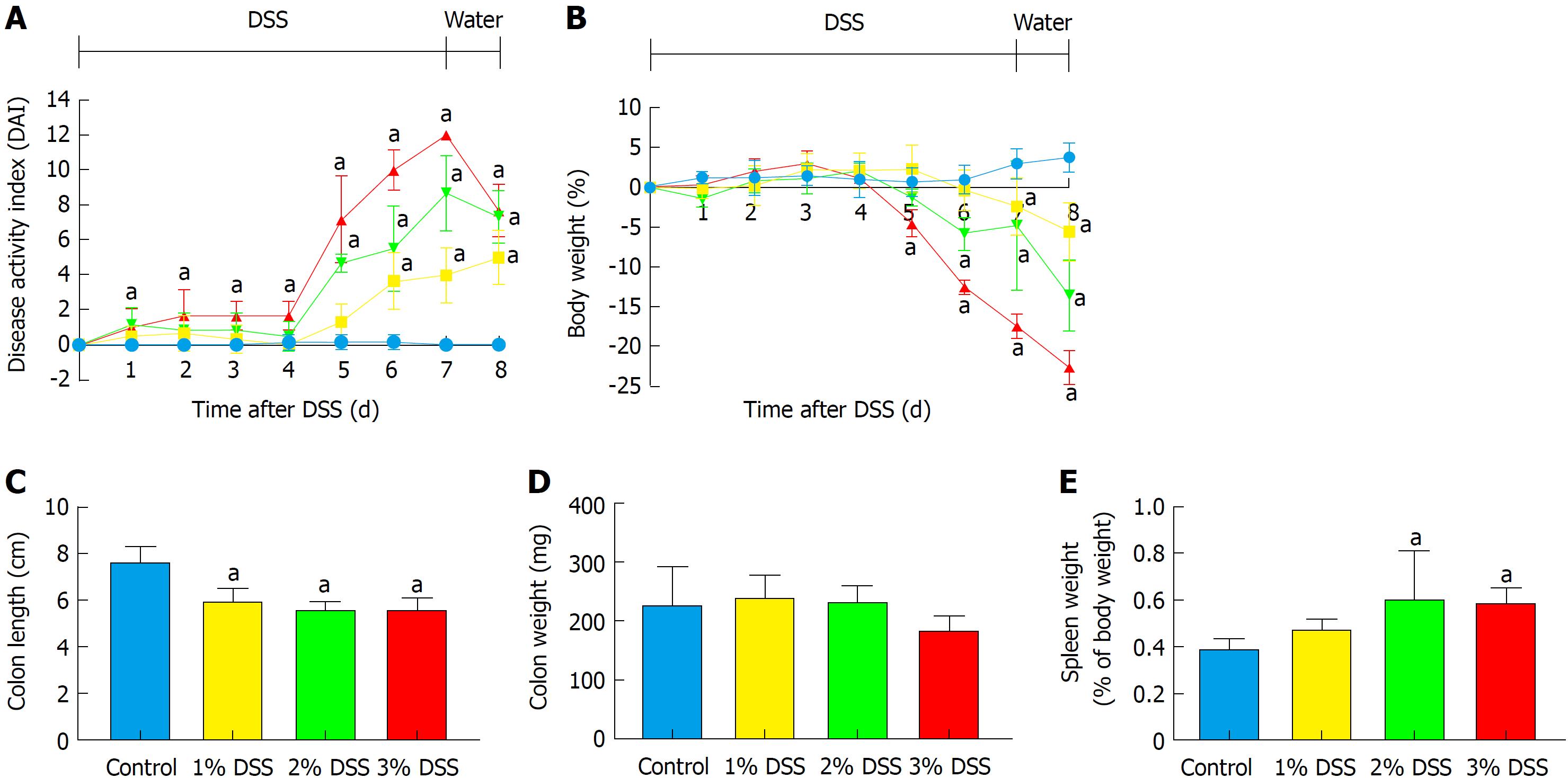
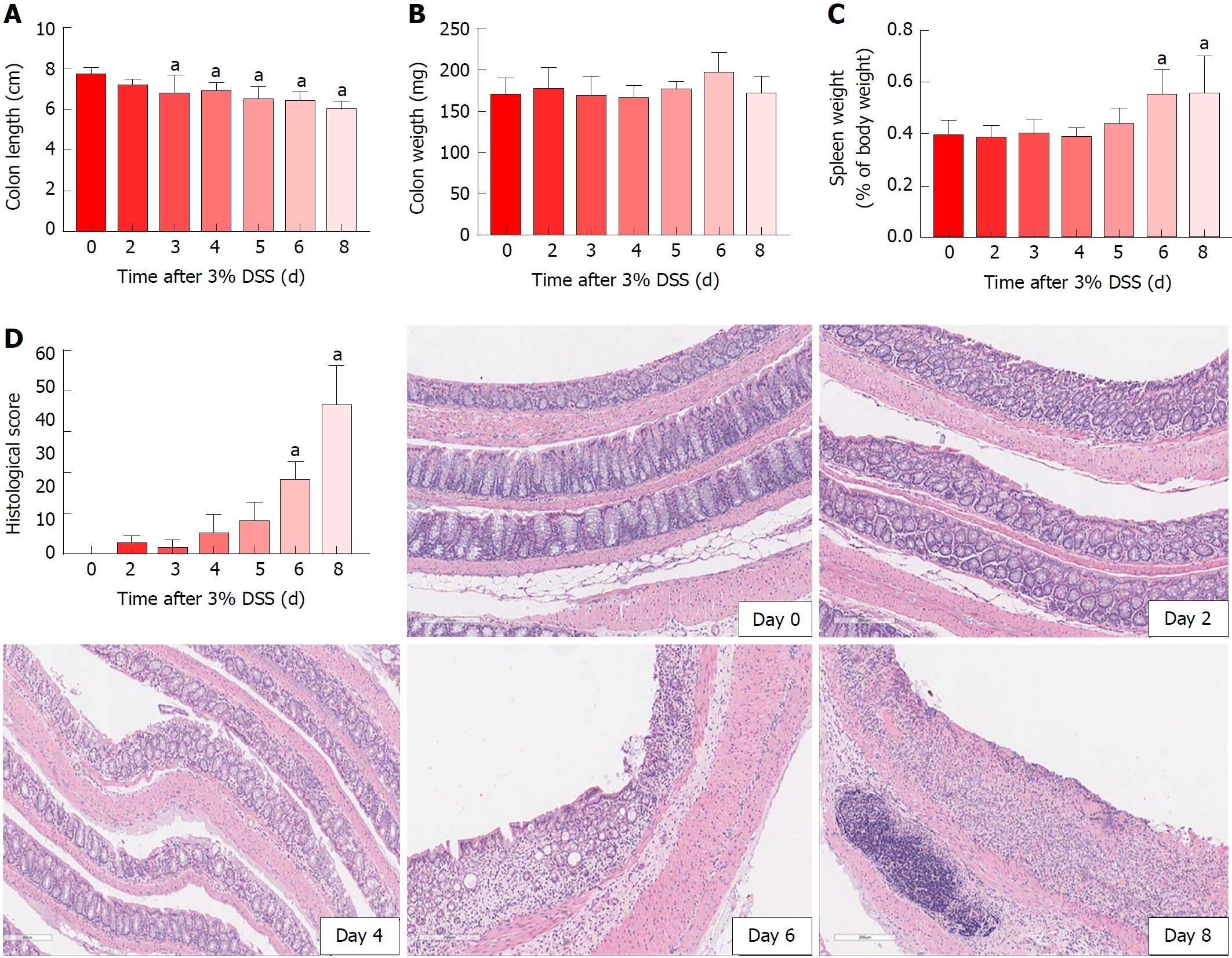
Acute DSS chemically induced colitis was evaluated at three dose levels of 1%, 2% and 3% for 7 d in the drinking water of mice and the clinical course was monitored and scored for the presence of bloody stools, watery diarrhea and weight loss for 8 d. In comparison to the control group, all three percentages of DSS in water resulted in progressive and increased clinical scores (Figure 1). The 1% DSS group exhibited weight loss starting on day 7, while the 2% DSS group showed variability in decreasing weight starting at day 6 (Figure 1). In comparison, mice that ingested 3% DSS showed prominent weight loss from day 5, reaching around -20% by day 8. For all DSS groups, colon lengths significantly decreased (P < 0.05) compared to control mice (Figure 1). The mean splenic weights significantly increased (P < 0.05) in mice ingesting 2% and 3% DSS compared to the control group, indicative of a robust systemic immune response (Figure 1). Histological scores were significantly higher (P < 0.05) in the 2% and 3% DSS groups, with clear evidence of destruction of crypts, loss of goblet cells, depletion of the epithelial barrier and infiltration of neutrophils and eosinophils at time of euthanasia (day 8) on HE staining (Figures 2 and 3). While there was no difference in colon weights amongst the groups compared to control mice, colon shortening was apparent in the 3% DSS cohort starting on day 3 (Figure 3). Splenic weights were significantly increased at day 6 and day 8, representing a systemic response one day before 3% DSS withdrawal and an increased histological inflammation (Figure 3). Overall, mice ingesting 3% DSS had greater clinical scores, weight loss, colon shortening, spleen weights and histological scores that led us to investigate the proteomic and immunological changes over time.
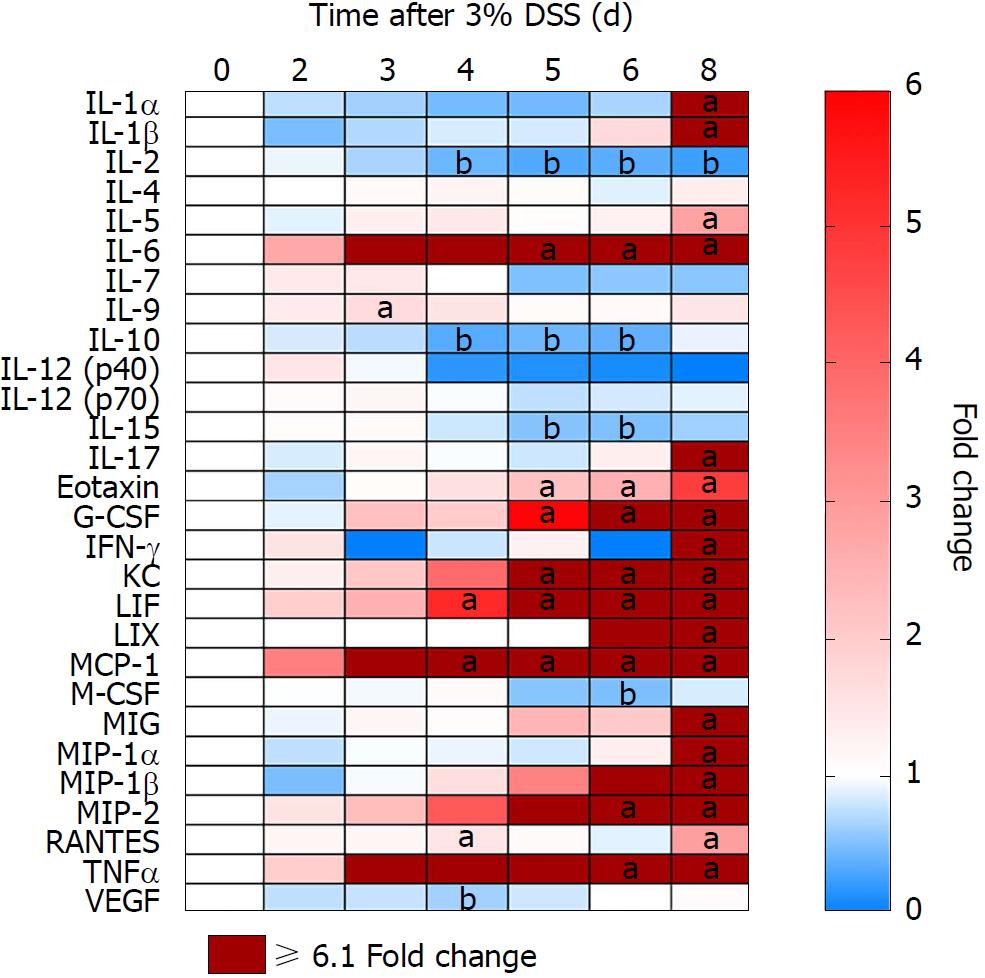
Proteomic changes following the introduction of 3% DSS in water were determined based on multiplex ELISA and showed a significant increase (P < 0.05, ANOVA compared to control) in expression of pro-inflammatory cytokines and chemokines: IL-1α, IL-1β, IL-5, IL-6, IL-9, IL-17, eotaxin, granulocyte-colony stimulating factor (G-CSF), interferon γ (IFN-γ), keratinocyte chemoattractant (KC), leukemia inhibitory factor (LIF), lipopolysaccharide-induced CXC chemokine (LIX), monocyte chemoattractant protein 1 (MCP-1), monokine induced by gamma interferon (MIG), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, regulated on activation, normal T cell expressed and secreted (RANTES) and TNFα starting at day 3 (Figure 4; Supplementary Figure 1 for raw data). Both pro and anti-inflammatory CCTF were elevated on day 8, when animals were ingesting water. There was a significant (P < 0.05) decrease in detection of IL-2, IL-10, IL-15, macrophage-colony stimulating factor (M-CSF) and vascular endothelial growth factor (VEGF) compared to control while animals were administered DSS 3%. We observed no changes in expression of IL-4, IL-7, IL12p40 and IL12p70 while animals ingested 3% DSS (Figure 4; Supplementary Figure 1). These results show that there are progressive inflammatory alterations in the colonic microenvironment that peaks one day after discontinuing DSS.
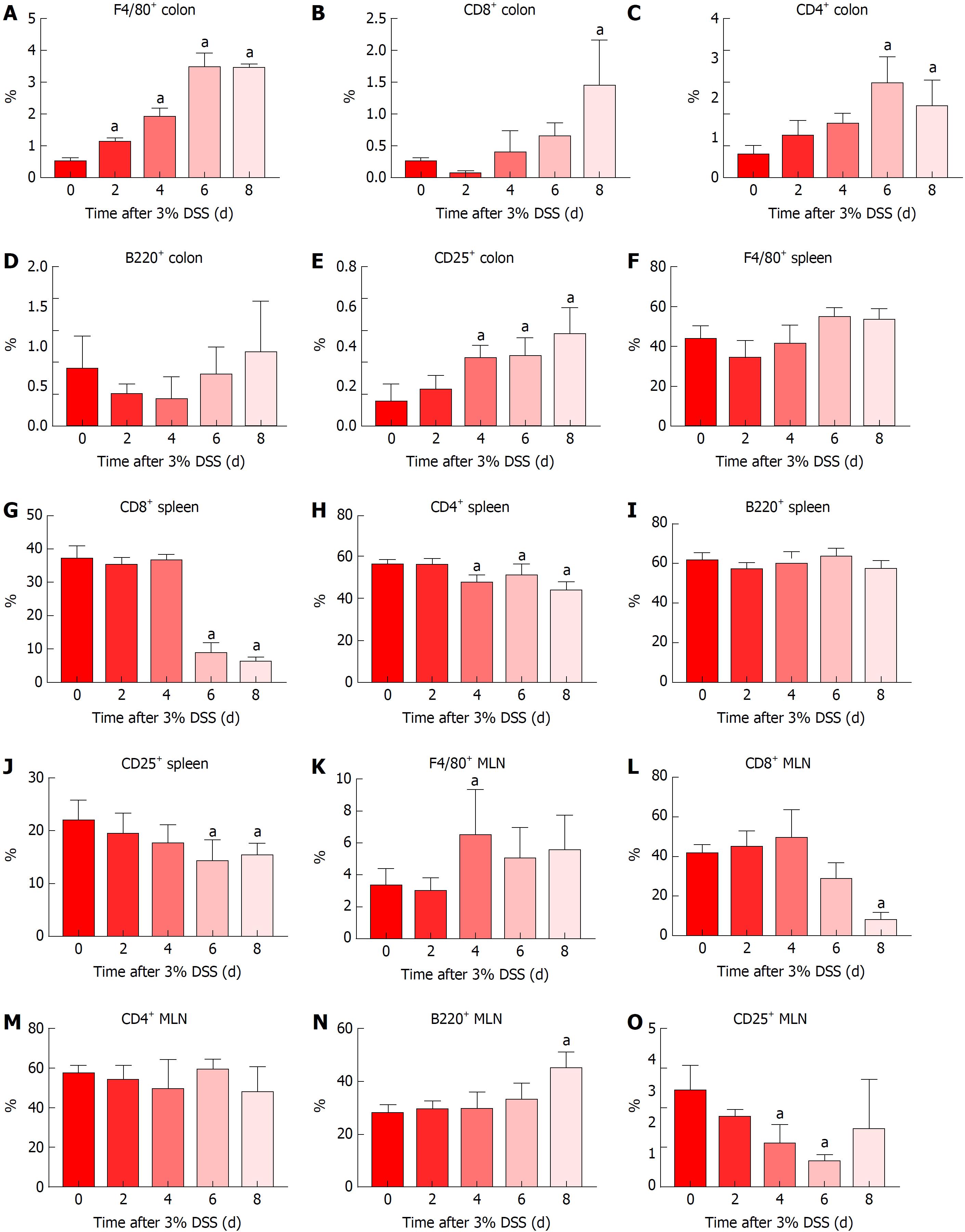
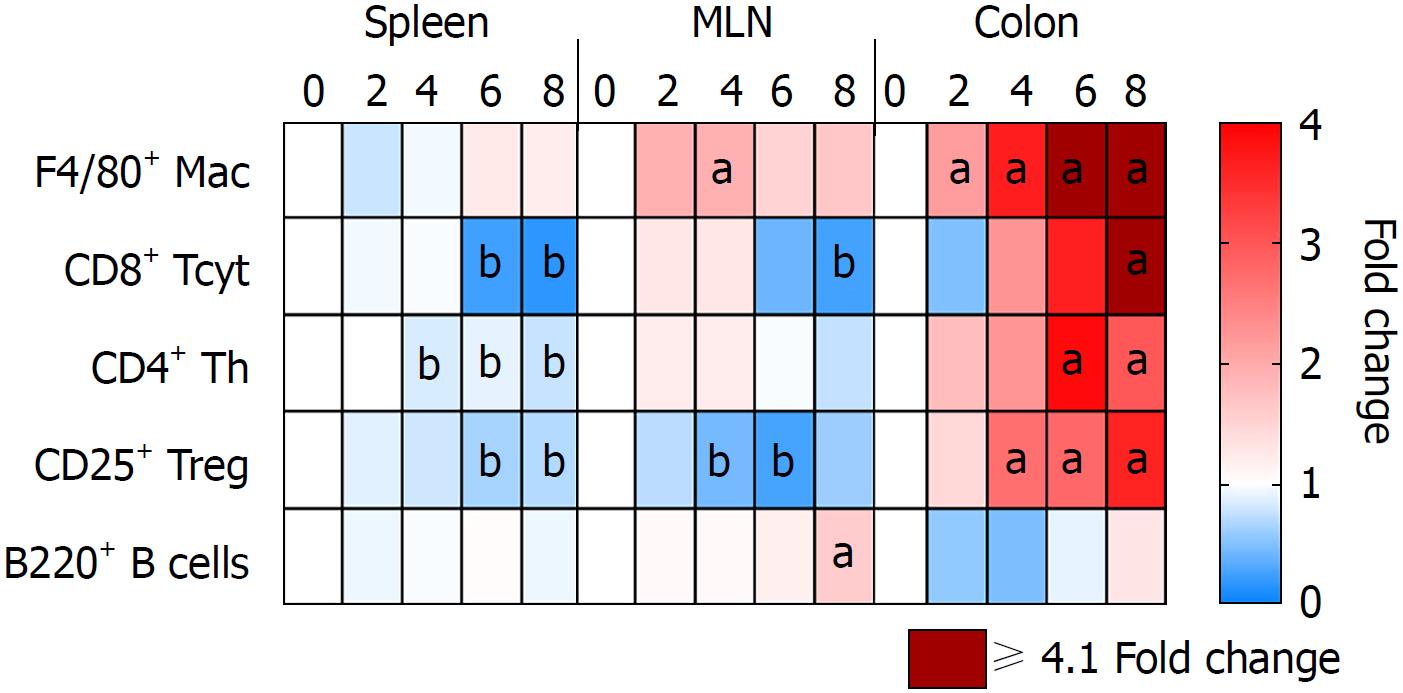
Immune cells population trafficking into the colon from the spleen and MLN were characterized from mice receiving 3% DSS by flow cytometry and IHC. The presence of macrophages (F4/80+) progressively increased (P < 0.05) in the colon from day 2 to day 8, while the spleen and MLN did not show differences when compared to control, besides a small increase in the MLN at day 4 (Figures 5 and 6). This observation would suggest that monocyte tropism to the colon probably originated from bone marrow instead of the secondary lymphoid organs. Cytotoxic T cells and Th cells were significantly elevated (P < 0.05) in the colon starting around day 6 and day 8, whereas Treg started to increase on day 4 as a countermeasure to the inflammatory environment in the colon. In comparison, Th cells were decreased only in the spleen from day 4 and forward, while both spleen and MLN demonstrated lower levels of Tcyt cells around day 6 and day 8. Treg were significantly (P < 0.05) decreased in the spleen and MLN starting on day 4 and day 6. B-cells (B220+) were significantly (P < 0.05) elevated in the MLN on day 8 after cessation of DSS, otherwise there was no changes compared to the control in the spleen and colon during the experiment. Figure 6 summarizes the fold changes in immune cell populations compared to day 0 (control) in the colon, spleen and MLN over the course of 8 d in this experiment and depict the trafficking of cells from secondary lymphoid tissues into the colon that resulted in an inflammatory response to 3% DSS. Detailed data on flow cytometry analysis can be found in supplementary figures 2 and 3. Images from HE and/or IHC analysis can be found in supplementary figures 4 and 5.
DSS is a chemically induced model of colitis characterized by a disruption of the epithelial barrier, resulting in microfloral substances entering the colonic mucosa and activating an innate immune response that produces local inflammatory factors[24]. It closely resembles human UC, which affects over 3.5 million people worldwide[25,26]. The acute tissue damage is characterized by a Th1/Th17 immune cell profile that leads to disease progression[15]. Previous studies have approached the analysis of acute DSS colitis by focusing on individual pathological features of the disease[9,15,16,19-21]. However, in the current study we demonstrate the temporal changes in clinical symptoms, histological features, immune cell population and proteomic response during the acute phase of DSS colitis.
We observed that the severity of experimental colitis was dependent on DSS concentration, and that clinical changes started as early as day 1, following initial ingestion. Increasing DSS concentration correlated with clinical disease severity, although on gross pathological examination all groups presented the same level of colon shortening. The difference between clinical and pathology suggest a mismatch (i.e., clinical disease severity does not correlate with histological scores) in the DSS colitis model. Our data contradicts the previous report[9], in which animals started to improve clinically and histologically after DSS withdrawal. These differences between studies may reflect the influence of the microbiome and/or the animals’ age, as previously reported in experimental DSS colitis[27,28]. In addition, it has been reported that UC patients in clinical and endoscopic based remission presenting with active histological inflammation possess a higher risk for clinical relapse[29,30]. In this way, our study may provide an understanding of the pathological and clinical response of severe human UC, with higher chances of relapsing and chronic disease. Since histological improvement could be seen as a new therapeutic approach and predictor of clinical relapse[31,32], the DSS clinical and molecular time course may be useful for evaluating novel therapeutic approaches with the goal of clinical pathological complete remission.
Morphological examination of the colon following 7 d of 3% DSS ingestion revealed that there is colonic shortening starting by day 3 and progressively decreases in size to day 8. In comparison, the weight of the colon does not change during DSS ingestion but splenic weight increases on day 6 and day 8, in agreement to previous studies in which splenic hypertrophy was observed in DSS colitis[33-36]. The increase in splenic size may represent congestion associated with an apparent proliferation of immune cells by HE staining. 3% DSS induces temporal changes in CCTF during the 8 d that can be segregated into four patterns: (pattern A) Progressive decreased expression of CCTF starting around day 4 (i.e., IL-2, IL-10 and IL-15); (pattern B) progressive increased expression of CCTF starting around day 4 (i.e., IL-6, Eotaxin, G-CSF, KC, LIF, MCP-1, MIP-2 and TNFα); (pattern C) increased expression of CCTF after stopping 3% DSS (i.e., day 8) of IL-1α, IL1β, IL-5, IL-17, IFN-γ, LIX, MIG, MIP-1a MIP-1b, and RANTES; and (pattern D) little or no change in CCTF from controls (i.e., IL-4, IL-7, IL-9, IL-12(p40), IL-12(p70), M-CSF and VEGF). The four patterns contain a mixture of pro-inflammatory and anti-inflammatory CCTF as well as chemoattractants associated with the influx of immune cell populations (i.e., neutrophils, eosinophils and macrophages) into the inflamed colon associated with loss of the normal epithelial barrier. For patterns A and B, changes in CCTF expression coincided with clinical worsening of colitis.
The decreased expression of IL-2, IL-10, and IL-15 starting on day 4 corresponds to the inflammatory response and progressive colonic damage. Decreased expression of IL-2 was previously seen in mononuclear cells derived from UC patient’s gut mucosa[37,38], as well as the disruption of the IL-2 gene in an animal model that exacerbated activation of lymphocytes, resembling auto-immunity[39,40]. In addition, the decreased expression of IL-10 would result in increased mucosal barrier disruption and increased TNFα and reactive oxygen species in the DSS model[41]. In the current study, there was a decrease in IL-15 starting at day 4, which should have attenuated colitis, based on results from DSS administration in the knockout mouse model[42]. It has been reported that the absence of IL-15 provokes a decrease in Foxp3 (Treg) and an increase in RORγt (Th17) by CD4+ T cells in the colon[43]. Such effect was not observed in our study, possibly due to a significant increase in IL-17 that may have contributed with other CCTFs to disease worsening.
Starting around day 4 of 3% DSS exposure, a progressive increase in IL-6, Eotaxin, G-CSF, KC, LIF, MCP-1, MIP-2 and TNFα was observed. IL-6 and TNFα interfere with epithelial tight junctions, increasing intestinal barrier permeability allowing for water loss and the para-cellular influx of molecules including the intrusion of pathogens that perpetuates the inflammatory process[44,45]. Elevation in IL-6 levels has an anti-apoptotic effect on lymphocytes, in addition to the increase of adhesion molecules that facilitate their migration to the gut[46,47]. Eotaxin, G-CSF, KC, LIF, MCP-1 and MIP-2 are chemokines associated with the influx of eosinophils, neutrophils and macrophages in the colon in active IBD[48-50]. Eotaxin is observed in DSS induced eosinophilic inflammation and promotes the recruitment of F4/80+CD11b+CCR2+Ly6Chigh inflammatory monocytes to the colon that correlates with eosinophilic inflammation[50]. MIP-2 has also been associated with increased inflammatory response in DSS induced colitis with increased myeloperoxidase activity and neutrophils infiltration in the colon and small intestine in a transgenic mouse model[51].
Increased expression of G-CSF, KC, LIF, and MCP-1 in DSS models has been associated with anti-apoptotic, anti-inflammatory phenotype with improvement in clinical scores, regulation of the immune response and morphological changes in the colon. G-CSF has been reported to reduce apoptosis of epithelial cells and along with other cytokines, helps in bacterial clearance through neutrophil recruitment to maintain the mucosal barrier integrity in IBD[52-54]. Treatment with recombinant G-CSF ameliorated DSS colitis by attenuating weight loss, stool score and shortening of the colon. In addition, inflammation, epithelial damage and cell apoptosis were attenuated in the rectum[54]. DSS acute colitis in KC deficient mice results in the increase of weight loss, bloody stools, inflammation and a moribund appearance, presenting higher histological scores but lower neutrophil infiltration compared to wild type (WT) animals[55]. LIF was found to be elevated in IBD patients[56] and has been shown to act in tissue damage by recruiting inflammatory cells to the injury site[57,58]. However, studies have shown that LIF also has an anti-inflammatory effect, stimulating repair and up-regulating Treg cells[58,59]. In our study, LIF expression increased on day 6 and could be responsible for modulating inflammation in the colon. In UC patients, the level of MCP-1 is directly related to disease activity[20,60-66]. It has been reported that intraperitoneal administration of MCP-1 significantly inhibited acute DSS colitis with lower clinical scores, increased survival, reduced weight loss, decreased production of IL-12 and IFN-γ associated with less inflammation[67]. MCP-1 may contribute to inflammation and colon shortening in our study, as well as inducing the elevation of pro-inflammatory CCTF by the end of 8 d. Although several CCTF included in Pattern B could be associated with improvements in clinical and pathological outcomes, in the current study, a predominant inflammatory microenvironment with disruption of the epithelium and infiltration of N and E into the lamina propria was observed in the colon.
Following cessation of DSS on day 7 (i.e., Pattern C), we detected significant increased expression of IL-1α, IL1β, IL-5, IL-17, IFN-γ, LIX, MIG, MIP-1a MIP-1b, and RANTES in the colon on Day 8. It is unclear how Pattern C relates to the removal of the DSS and the apparent rebound of primarily pro-inflammatory CCTF in the microenvironment. IL-1α and IL-1β can be potent chemoattractants for neutrophils and macrophages into the microenvironment by the induction and propagation of the inflammatory response[68]. IL-1α and IL-1β can induce increases in pro-inflammatory chemokines as well as cell adhesion molecules, activate macrophages, dendritic cells and neutrophils, and support Th17 cells’ differentiation[69,70]. IL-5 is characteristic of a Th2 immune response in UC that stimulates eosinophil growth, development, survival and activation, mobilizing them from the bone marrow to the peripheral blood. IL-5 along with Eotaxin serve as chemoattractants of E to the gut[71-75]. IL17 may also play a dual role in DSS colitis models as it has been reported to stimulate the production of matrix metalloproteinase, increase the expression of other pro-inflammatory factors (e.g., IL-6, IL-1β, TNFα, KC, MCP-1, MIP-2, GM-CSF), and to be involved in the proliferation, maturation and chemotaxis of N[76-80]. In IL-17A deficient mice, the DSS colitis model is associated with improved survival and histological scores with less epithelial damage and immune cell infiltration in the intestine, when compared to WT mice[13]. However, studies have shown IL-17 to stabilize the epithelial barrier and to aggravate colitis when absent in the animal[76,81,82]. In the current study, it is unclear if IL-17 contributes to the inflammatory responses in the colon or improvement in the clinical score at the end of 8 d.
IFN-γ expression was significantly increased at day 8 and is highly expressed by CD8+ T cells from IBD patients, when in contact with colonic epithelial cells[83]. IFN-γ stimulates the disruption of the intestinal epithelial barrier and supports the exacerbated immune response in IBD[44,84]. It is also essential for DSS colitis model, since IFN-γ-/- knockout mice do not develop colitis when challenged with DSS[84]. In this study, high levels of Tcyt cells and F4/80 macrophages were found in the colon that may be responsible for the increased levels of IFN-γ and the observed inflammatory response. LIX was found to be elevated in UC patients and contributes to the inflammatory response in DSS colitis. Of note, pre-treatment of mice with antisense oligonucleotides to LIX in the DSS colitis model reduces neutrophils’ infiltration and the severity of the disease[85]. MIG can act as a chemoattractant for activated Tcyt cells, E and natural killer (NK) cells, along with having an angiostatic effect on endothelial cells by inhibiting cell division in colitis models[86,87]. MIP1-α and MIP1-β are chemoattractants for T cells into the lamina propria that can lead to mucosal damage and worsening of colitis[88,89]. Finally, RANTES has been shown to be elevated in chronic experimental colitis[90] and in the colonic mucosa of IBD patients, supporting both innate and adaptive immune responses[61,90-93]. Taken together, Pattern C appears to be associated with a rebound increased expression of inflammatory CCTF that contributed to colon pathology and higher histological scores.
Pattern D includes IL-4, IL-7, IL-9, IL-12(p40), IL-12(p70), M-CSF and VEGF that do not significantly change or were elevated at a random single time point over 8 d. IL-9 was elevated at day 3 coinciding when colon shortening was first detected. T cells expressing IL-9 are found in the intestinal mucosa in experimental colitis and UC patients. IL-9 is responsible for disruption of the intestinal barrier and the impairment of mucosal tissue repair through suppression of epithelial cell proliferation[94-96], which relates to the mucosal injury during the disease course. M-CSF, which mainly induces M2 macrophage phenotype[97-99], that was significantly (P < 0.05) decreased at day 6, when the pro-inflammatory CCTFs in Pattern B were increasing, associated with a possible predominance of M1 macrophages. M-CSF has also been proposed as an alternative therapy in treating UC and DSS colitis[100,101]. VEGF was found downregulated at day 4 and is usually found elevated in DSS model, however, it is also associated with lymphangiogenesis, which would aid in the clearance of interstitial fluid and immune cells from the colon[102-104]. With low VEGF levels, there could be impaired drainage function and lymphatic obstruction[103,105], leading to the accumulation of immune cells in the gut and disease worsening. In summary, the CCTF profiles observed following DSS are consistent of a pro-inflammatory microenvironment. The changes in CCTF over time serve as chemoattractants for neutrophils, eosinophils and macrophages as well as disrupting the integrity of the epithelial barrier and production of mucin. The inflammatory response in the colon leads to a clinical course that includes a malabsorption like syndrome accompanied by weight loss, bloody stools and diarrhea.
Analysis of immune cell populations revealed a progressive accumulation of macrophages and T cells (Th, Tcyt and Treg cells) in the colon compared to controls. It has been previously shown[16] that colonic CD3+ T cells and F4/80+ macrophages were upregulated in later time points when evaluating acute and chronic DSS colitis, as compared to the current study. In addition, splenic and MLN F4/80+ population were highly elevated whereas we did observe little or no change compared to control mice. Another study has reported that intestinal inflammation in UC presents as the initial fast response with increased number of macrophages originating from tissue-resident or infiltrating systemic macrophages in the intestinal mucosa[106]. The early arrival of macrophages in the gut at day 2 contributes to the initiation of inflammation coinciding with increase in pro-inflammatory CCTF and translating into clinical symptoms.
In the current study, T-cell phenotypes in the colon begin to increase 4 d after initiation of 3% DSS, which contributes to the perpetuation of inflammation and the high levels of CCTF seen at day 8. Decreased levels of Th and Tcyt cells were observed in the spleen, and to lesser extent in the MLN, starting at day 6 when compared to controls. Moreover, there were no significant differences in percentage of B220+ cells in the colon or lymphoid organs compared to control levels, other than an increase in the MLN at day 8, suggesting the transition to a chronic state. Although T and B cells are not required for the development of DSS colitis[6], in this study T cells appear to contribute to the activation of the inflammatory response in the colon. In addition, Treg cells seem to be leaving the secondary lymphoid organs migrating towards the colon in an attempt to contain the exacerbated immune response.
There have been few reports describing the temporal distribution of immune cells along with proteomic changes in the colon in DSS colitis model[16,17]. In this study we observed that severity of colitis is dependent on DSS concentration, while presenting discrepancies amongst clinical and pathological results. When mice were administered 3% DSS, we observed increased clinical and histological scores that were accompanied by changes in CCTF and immune cell infiltration in the colon, with important participation of the secondary lymphoid organs. One limitation of this study is that DSS colitis was induced in C56Bl/6 mice from a single vendor and it is unclear if the same mouse strain containing a different microbiota may have influenced the temporal clinical, proteomic and pathological changes we observed in the current study. Further investigations are needed to determine the role of the gut flora in the development of colitis and the response to novel therapeutic interventions that could translate to clinical trials. Furthermore, acknowledging the time frame where these factors play a role in developing novel therapies for treating ulcerative colitis.
Ulcerative colitis (UC) is an inflammatory bowel disease that affects the colon and the rectum, being characterized by uncontrolled immune response and inflammation. There is no specific cause for this disease and no current treatment that provides sustained remission. The animal model of colitis induced by dextran sulfate sodium (DSS) is largely used as a tool to better investigate human UC. Although not completely understood, DSS induces an uncontrolled immune response through disruption of the epithelial layer, providing a higher access of antigens to the colonic mucosa, this way perpetuating inflammation and tissue destruction.
There is no current study providing a detailed integrative temporal analysis of DSS-induced acute colitis regarding clinical symptoms, proteomics, immune cell profile and histology. Understanding the interaction of these factors may contribute to the research of novel UC therapies.
The aim of this study was to compare different concentrations of DSS in the induction of acute colitis, followed by a temporal analysis of clinical symptoms, colon proteomics, immune cell profile and histology of the most characteristic presentation of colitis amongst the different DSS concentrations. The changes seen throughout the 8 d may provide a clearer understanding of the DSS model mechanisms.
1%, 2% and 3% DSS in drinking water was used for the induction of acute colitis. Clinical symptoms were daily scored for weight loss, stool consistency and blood in the stool. After 8 d, colon, spleen and mesenteric lymph nodes (MLN) were collected. Histological scores were evaluated through HE staining and grading of colonic samples for inflammation, extent, regeneration, crypt damage and percent of involvement. Colon proteomics was analyzed through multiplex ELISA for 3% DSS at different time points, in addition to immune cell profiling of the colon, spleen and MLN through immunohistochemistry and flow cytometry for B220+, CD4+, CD8+, CD25+ and F4/80+ cells.
Severity of colitis is related to the increase in DSS concentration. When analyzing 3% DSS-induced colitis, worsening of histological inflammation agrees with an increase of immune cells’ influx to the colon and changes in the pro- and anti-inflammatory cytokines colonic profile. Macrophages are the first ones to respond to the damage caused by DSS, followed by changes in the colonic cytokine profile and influx of CD25+ T cells. Next, there is an increase in colonic CD4+ and CD8+ T cells and the highest level of pro-inflammatory cytokines is seen at day 8. Levels of T cells are progressively decreased in the spleen and MLN, while worsening of clinical symptoms corresponds with the progressive increase in histological inflammation, with exception of day 8.
Our study demonstrates the correlated temporal changes of clinical, proteomic, immunological and histological characteristics of DSS-induced acute colitis. There is an important initial response by the innate immune system, mainly coordinated by macrophages, followed by increasing inflammation, further tissue damage and influx of T cells. T cells may be leaving the secondary lymphoid organs progressively towards the gut, as a response to the changes in colonic cytokine levels. There is a mixed response of pro- and anti-inflammatory cytokines in the colon, with the highest increase occurring after DSS withdrawal. Interestingly, amelioration of clinical symptoms is seen on day 8, demonstrating a mismatch to the histological/immunological/proteomic worsening of the disease. Since histological inflammation is seen in UC patients with endoscopic and clinical remission, this model could be used as a tool for the development of novel therapies targeting complete remission and prevention of disease relapse.
Our study demonstrates that no individual factor develops this disease model, but rather a coordination between anti- and pro-inflammatory cytokines. Therefore, researchers should seriously consider a temporal analysis before investigating new therapies. The disease course here described would be highly recommended for the study of novel treatments aiming resolution of histological inflammation during disease remission. Further temporal analysis of DSS-induced chronic colitis would add to a better understanding of this animal model.
This work was supported by the Intramural Research Programs of the Clinical Center, the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health and CAPES (Coordination for the Training of Higher Education Personnel Ministry of Education) from Brazil.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Keshteli AH, Suzuki H S- Editor: Wang XJ L- Editor: A E- Editor: Bian YN
| 1. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 992] [Cited by in F6Publishing: 979] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2894] [Cited by in F6Publishing: 3243] [Article Influence: 190.8] [Reference Citation Analysis (11)] |
| 3. | Low D, Nguyen DD, Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther. 2013;7:1341-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci. 2012;105:263-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Kiesler P, Fuss IJ, Strober W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol Gastroenterol Hepatol. 2015;1:154-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 461] [Article Influence: 51.2] [Reference Citation Analysis (1)] |
| 6. | Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 15.25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 1195] [Article Influence: 119.5] [Reference Citation Analysis (1)] |
| 7. | Gonçalves FC, Schneider N, Mello HF, Passos EP, Meurer L, Cirne-Lima E, Paz AH. Characterization of Acute Murine Dextran Sodium Sulfate (DSS) Colitis: Severity of InFammation is Dependent on the DSS Molecular Weight and Concentration. Acta Scientiae Veterinariae. 2013;41:1-9. [Cited in This Article: ] |
| 8. | Padua D, Vu JP, Germano PM, Pisegna JR. The Role of Neuropeptides in Mouse Models of Colitis. J Mol Neurosci. 2016;59:203-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, Merlin D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One. 2009;4:e6073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1733] [Cited by in F6Publishing: 1792] [Article Influence: 52.7] [Reference Citation Analysis (7)] |
| 11. | Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261-1270. [PubMed] [Cited in This Article: ] |
| 12. | Sands BE, Kaplan GG. The role of TNFalpha in ulcerative colitis. J Clin Pharmacol. 2007;47:930-941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377:12-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808-1813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 590] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 16. | Hall LJ, Faivre E, Quinlan A, Shanahan F, Nally K, Melgar S. Induction and activation of adaptive immune populations during acute and chronic phases of a murine model of experimental colitis. Dig Dis Sci. 2011;56:79-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 625] [Article Influence: 52.1] [Reference Citation Analysis (1)] |
| 18. | Al-Haddad S, Riddell RH. The role of eosinophils in inflammatory bowel disease. Gut. 2005;54:1674-1675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328-G1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Fang K, Grisham MB, Kevil CG. Application of Comparative Transcriptional Genomics to Identify Molecular Targets for Pediatric IBD. Front Immunol. 2015;6:165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Fang K, Bruce M, Pattillo CB, Zhang S, Stone R 2nd, Clifford J, Kevil CG. Temporal genomewide expression profiling of DSS colitis reveals novel inflammatory and angiogenesis genes similar to ulcerative colitis. Physiol Genomics. 2011;43:43-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Gonçalves Fda C, Schneider N, Pinto FO, Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP, Cirne-Lima EO, Meurer L. Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World J Gastroenterol. 2014;20:18228-18239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 51] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 747] [Cited by in F6Publishing: 891] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 24. | Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1157] [Cited by in F6Publishing: 1696] [Article Influence: 188.4] [Reference Citation Analysis (0)] |
| 26. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1030] [Cited by in F6Publishing: 1012] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 27. | Roy U, Gálvez EJC, Iljazovic A, Lesker TR, Błażejewski AJ, Pils MC, Heise U, Huber S, Flavell RA, Strowig T. Distinct Microbial Communities Trigger Colitis Development upon Intestinal Barrier Damage via Innate or Adaptive Immune Cells. Cell Rep. 2017;21:994-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 28. | Park YH, Kim N, Shim YK, Choi YJ, Nam RH, Choi YJ, Ham MH, Suh JH, Lee SM, Lee CM. Adequate Dextran Sodium Sulfate-induced Colitis Model in Mice and Effective Outcome Measurement Method. J Cancer Prev. 2015;20:260-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Lobatón T, Bessissow T, Ruiz-Cerulla A, De Hertogh G, Bisschops R, Guardiola J, Van Assche G, Vermeire S, Ferrante M. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: A prospective multicenter study. United European Gastroenterol J. 2018;6:765-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Narang V, Kaur R, Garg B, Mahajan R, Midha V, Sood N, Sood A. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intest Res. 2018;16:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Azad S, Sood N, Sood A. Biological and histological parameters as predictors of relapse in ulcerative colitis: a prospective study. Saudi J Gastroenterol. 2011;17:194-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8:1582-1597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 225] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 33. | Hong HS, Hwang DY, Park JH, Kim S, Seo EJ, Son Y. Substance-P alleviates dextran sulfate sodium-induced intestinal damage by suppressing inflammation through enrichment of M2 macrophages and regulatory T cells. Cytokine. 2017;90:21-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Da Silva AP, Pollett A, Rittling SR, Denhardt DT, Sodek J, Zohar R. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J Cell Physiol. 2006;208:629-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O, Sartor RB. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest. 2000;105:469-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Axelsson LG, Landström E, Bylund-Fellenius AC. Experimental colitis induced by dextran sulphate sodium in mice: beneficial effects of sulphasalazine and olsalazine. Aliment Pharmacol Ther. 1998;12:925-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Fiocchi C, Hilfiker ML, Youngman KR, Doerder NC, Finke JH. Interleukin 2 activity of human intestinal mucosa mononuclear cells. Decreased levels in inflammatory bowel disease. Gastroenterology. 1984;86:734-742. [PubMed] [Cited in This Article: ] |
| 38. | Van Damme N, De Keyser F, Demetter P, Baeten D, Mielants H, Verbruggen G, Cuvelier C, Veys EM, De Vos M. The proportion of Th1 cells, which prevail in gut mucosa, is decreased in inflammatory bowel syndrome. Clin Exp Immunol. 2001;125:383-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 710] [Cited by in F6Publishing: 692] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 40. | Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1295] [Cited by in F6Publishing: 1250] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 41. | Li B, Alli R, Vogel P, Geiger TL. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014;7:869-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 42. | Yoshihara K, Yajima T, Kubo C, Yoshikai Y. Role of interleukin 15 in colitis induced by dextran sulphate sodium in mice. Gut. 2006;55:334-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Tosiek MJ, Fiette L, El Daker S, Eberl G, Freitas AA. IL-15-dependent balance between Foxp3 and RORγt expression impacts inflammatory bowel disease. Nat Commun. 2016;7:10888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol. 2017;4:33-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 401] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 45. | Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 46. | Allocca M, Jovani M, Fiorino G, Schreiber S, Danese S. Anti-IL-6 treatment for inflammatory bowel diseases: next cytokine, next target. Curr Drug Targets. 2013;14:1508-1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Katsanos KH, Papadakis KA. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver. 2017;11:455-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 48. | Chen W, Paulus B, Shu D, Wilson , Chadwick V. Increased serum levels of eotaxin in patients with inflammatory bowel disease. Scand J Gastroenterol. 2001;36:515-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Ahrens R, Waddell A, Seidu L, Blanchard C, Carey R, Forbes E, Lampinen M, Wilson T, Cohen E, Stringer K. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J Immunol. 2008;181:7390-7399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Waddell A, Ahrens R, Steinbrecher K, Donovan B, Rothenberg ME, Munitz A, Hogan SP. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J Immunol. 2011;186:5993-6003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Ohtsuka Y, Sanderson IR. Dextran sulfate sodium-induced inflammation is enhanced by intestinal epithelial cell chemokine expression in mice. Pediatr Res. 2003;53:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Martins A, Han J, Kim SO. The multifaceted effects of granulocyte colony-stimulating factor in immunomodulation and potential roles in intestinal immune homeostasis. IUBMB Life. 2010;62:611-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Smith AM, Rahman FZ, Hayee B, Graham SJ, Marks DJ, Sewell GW, Palmer CD, Wilde J, Foxwell BM, Gloger IS. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206:1883-1897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 54. | Kudo T, Matsumoto T, Nakamichi I, Yada S, Esaki M, Jo Y, Ohji Y, Yao T, Iida M. Recombinant human granulocyte colony-stimulating factor reduces colonic epithelial cell apoptosis and ameliorates murine dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2008;43:689-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Shea-Donohue T, Thomas K, Cody MJ, Aiping Zhao, Detolla LJ, Kopydlowski KM, Fukata M, Lira SA, Vogel SN. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 391] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 57. | Guimbaud R, Abitbol V, Bertrand V, Quartier G, Chauvelot-Moachon L, Giroud J, Couturier D, Chaussade DC. Leukemia inhibitory factor involvement in human ulcerative colitis and its potential role in malignant course. Eur Cytokine Netw. 1998;9:607-612. [PubMed] [Cited in This Article: ] |
| 58. | Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015;26:533-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 304] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 59. | Metcalfe SM. LIF in the regulation of T-cell fate and as a potential therapeutic. Genes Immun. 2011;12:157-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Li YW, Yang CQ, Xiao YL, Li J, Xie CX, Zhang SH, Yu Q, Wang HL, Lu WM, Chen MH. The -A2518G polymorphism in the MCP-1 gene and inflammatory bowel disease risk: A meta-analysis. J Dig Dis. 2015;16:177-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Günaltay S, Kumawat AK, Nyhlin N, Bohr J, Tysk C, Hultgren O, Hultgren Hörnquist E. Enhanced levels of chemokines and their receptors in the colon of microscopic colitis patients indicate mixed immune cell recruitment. Mediators Inflamm. 2015;2015:132458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Grimm MC, Elsbury SK, Pavli P, Doe WF. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol. 1996;59:804-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol. 2003;199:28-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 64. | Reinecker HC, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995;108:40-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 195] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 66. | Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. 1999;155:331-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 213] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Maharshak N, Hart G, Ron E, Zelman E, Sagiv A, Arber N, Brazowski E, Margalit R, Elinav E, Shachar I. CCL2 (pM levels) as a therapeutic agent in Inflammatory Bowel Disease models in mice. Inflamm Bowel Dis. 2010;16:1496-1504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835-4843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 69. | Lei-Leston AC, Murphy AG, Maloy KJ. Epithelial Cell Inflammasomes in Intestinal Immunity and Inflammation. Front Immunol. 2017;8:1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 70. | Aguilera M, Darby T, Melgar S. The complex role of inflammasomes in the pathogenesis of Inflammatory Bowel Diseases - lessons learned from experimental models. Cytokine Growth Factor Rev. 2014;25:715-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Bamias G, Cominelli F. Role of type 2 immunity in intestinal inflammation. Curr Opin Gastroenterol. 2015;31:471-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 833] [Cited by in F6Publishing: 810] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 73. | Fulkerson PC, Rothenberg ME. Origin, regulation and physiological function of intestinal oeosinophils. Best Pract Res Clin Gastroenterol. 2008;22:411-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Mishra A, Hogan SP, Brandt EB, Wagner N, Crossman MW, Foster PS, Rothenberg ME. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem. 2002;277:4406-4412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 470] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 76. | Owaga E, Hsieh RH, Mugendi B, Masuku S, Shih CK, Chang JS. Th17 Cells as Potential Probiotic Therapeutic Targets in Inflammatory Bowel Diseases. Int J Mol Sci. 2015;16:20841-20858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 77. | Xu XR, Liu CQ, Feng BS, Liu ZJ. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2014;20:3255-3264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 150] [Cited by in F6Publishing: 166] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 78. | Chen L, Zou Y, Peng J, Lu F, Yin Y, Li F, Yang J. Lactobacillus acidophilus suppresses colitis-associated activation of the IL-23/Th17 axis. J Immunol Res. 2015;2015:909514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 79. | Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1119] [Cited by in F6Publishing: 1151] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 80. | Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513-3521. [PubMed] [Cited in This Article: ] |
| 81. | Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 339] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 82. | Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 385] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 83. | Bisping G, Lügering N, Lütke-Brintrup S, Pauels HG, Schürmann G, Domschke W, Kucharzik T. Patients with inflammatory bowel disease (IBD) reveal increased induction capacity of intracellular interferon-gamma (IFN-gamma) in peripheral CD8+ lymphocytes co-cultured with intestinal epithelial cells. Clin Exp Immunol. 2001;123:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Kita M, Ueda Y, Iwakura Y. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 85. | Kwon JH, Keates AC, Anton PM, Botero M, Goldsmith JD, Kelly CP. Topical antisense oligonucleotide therapy against LIX, an enterocyte-expressed CXC chemokine, reduces murine colitis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1075-G1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Egesten A, Eliasson M, Olin AI, Erjefält JS, Bjartell A, Sangfelt P, Carlson M. The proinflammatory CXC-chemokines GRO-alpha/CXCL1 and MIG/CXCL9 are concomitantly expressed in ulcerative colitis and decrease during treatment with topical corticosteroids. Int J Colorectal Dis. 2007;22:1421-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 284] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 88. | Li K, Wang B, Sui H, Liu S, Yao S, Guo L, Mao D. Polymorphisms of the macrophage inflammatory protein 1 alpha and ApoE genes are associated with ulcerative colitis. Int J Colorectal Dis. 2009;24:13-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Pender SL, Chance V, Whiting CV, Buckley M, Edwards M, Pettipher R, MacDonald TT. Systemic administration of the chemokine macrophage inflammatory protein 1alpha exacerbates inflammatory bowel disease in a mouse model. Gut. 2005;54:1114-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AE, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Tahara T, Shibata T, Okubo M, Ishizuka T, Kawamura T, Yamashita H, Nakamura M, Nakagawa Y, Nagasaka M, Arisawa T. Effect of RANTES gene promoter genotypes in patients with ulcerative colitis. Biomed Rep. 2014;2:602-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Ansari N, Abdulla J, Zayyani N, Brahmi U, Taha S, Satir AA. Comparison of RANTES expression in Crohn’s disease and ulcerative colitis: an aid in the differential diagnosis? J Clin Pathol. 2006;59:1066-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 963] [Cited by in F6Publishing: 1017] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 94. | Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, Lehr HA, Wirtz S, Vieth M, Waisman A. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15:676-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 293] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 95. | Hufford MM, Kaplan MH. A gut reaction to IL-9. Nat Immunol. 2014;15:599-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Leake I. IBD. TH9 cells might have a role in the pathogenesis of ulcerative colitis. Nat Rev Gastroenterol Hepatol. 2014;11:455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Zwicker S, Bureik D, Bosma M, Martinez GL, Almer S, Boström EA. Receptor-Type Protein-Tyrosine Phosphatase ζ and Colony Stimulating Factor-1 Receptor in the Intestine: Cellular Expression and Cytokine- and Chemokine Responses by Interleukin-34 and Colony Stimulating Factor-1. PLoS One. 2016;11:e0167324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Klebl FH, Olsen JE, Jain S, Doe WF. Expression of macrophage-colony stimulating factor in normal and inflammatory bowel disease intestine. J Pathol. 2001;195:609-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Makiyama K, Tomonaga M, Nakamuta K, Oda H, Itsuno M, Hara K. Serum concentration of macrophage colony stimulating factor (M-CSF) in patients with inflammatory bowel disease. Gastroenterol Jpn. 1993;28:740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 100. | Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, Chang MW, Beckman SK, Cook AD, Hamilton JA. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol. 2012;188:5752-5765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 389] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 101. | Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 905] [Cited by in F6Publishing: 976] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 102. | Linares PM, Gisbert JP. Role of growth factors in the development of lymphangiogenesis driven by inflammatory bowel disease: a review. Inflamm Bowel Dis. 2011;17:1814-1821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 103. | Becker F, Potepalov S, Shehzahdi R, Bernas M, Witte M, Abreo F, Traylor J, Orr WA, Tsunoda I, Alexander JS. Downregulation of FoxC2 Increased Susceptibility to Experimental Colitis: Influence of Lymphatic Drainage Function? Inflamm Bowel Dis. 2015;21:1282-1296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158-3167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 105. | Becker F, Yi P, Al-Kofahi M, Ganta VC, Morris J, Alexander JS. Lymphatic dysregulation in intestinal inflammation: new insights into inflammatory bowel disease pathomechanisms. Lymphology. 2014;47:3-27. [PubMed] [Cited in This Article: ] |
| 106. | Kühl AA, Erben U, Kredel LI, Siegmund B. Diversity of Intestinal Macrophages in Inflammatory Bowel Diseases. Front Immunol. 2015;6:613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |