Published online Jun 14, 2018. doi: 10.3748/wjg.v24.i22.2381
Peer-review started: February 12, 2018
First decision: February 24, 2018
Revised: March 8, 2018
Accepted: April 16, 2018
Article in press: April 15, 2018
Published online: June 14, 2018
Processing time: 119 Days and 19.4 Hours
To investigate the relationship between hypoxia-inducible factor-1α (HIF-1α), prolyl 4-hydroxylase beta (P4HB) expression, and clinicopathologic parameters, as well as the prognostic value of these genes for patients with gastric cancer (GC).
Hypoxia is a critical factor that shapes the GC microenvironment. In previous reports, we have demonstrated that P4HB is a potential target of HIF-1α. In the present study, gene expression profiling interactive analysis (GEPIA) was used to analyze the relationship between P4HB and hypoxia-associated genes. To this end, 428 GC tissue samples were used to analyze the expression of HIF-1α and P4HB via immunohistochemical staining. Patient samples were classified as having weak-expression or over-expression both in terms of HIF-1α and P4HB. Correlations between biomarkers and clinicopathological factors were analyzed to predict survival.
P4HB demonstrated a positive correlation with hypoxia-associated genes (P < 0.05). HIF-1α and P4HB overexpression have a significant correlation with TNM staging (χ2 = 23.32, P = 0.00; χ2 = 65.64, P = 0.00) and peritoneum cavity metastasis (χ2 = 12.67, P = 0.00; χ2 = 39.29, P = 0.00). In univariate analysis, patients with a high HIF-1α expression trend had a shorter disease-free survival (DFS: 44.80 mo vs 22.06 mo) and overall survival (OS: 49.58 mo vs 39.92 mo). P4HB overexpression reflected similar results: patients with over-expression of P4HB had a shorter survival time than those with weak-expression (DFS: 48.03 mo vs 29.64 mo, OS: 52.48 mo vs 36.87 mo). Furthermore, HIF-1α is also a clinicopathological predictor of dismal prognosis according to multivariate analysis (DFS, 95%CI: 0.52-0.88, P < 0.00; OS, 95%CI: 0.50-0.85, P < 0.00). However, P4HB was meaningful in DFS (95%CI: 0.58-1.00, P < 0.05) but not in OS (95%CI: 0.72-1.23, P > 0.05).
Overexpression of HIF-1α and P4HB is associated with poor prognosis in patients with GC. Thus, these genes may be potential prognostic biomarker candidates in GC.
Core tip: The progression of gastric cancer is closely related to the hypoxic tumor microenvironment. In the present study, we found the expression levels of prolyl 4-hydroxylase beta (P4HB) and hypoxia-inducible factor-1α (HIF-1α) were significantly increased in gastric cancer tissue, and patients with high P4HB and HIF-1α expression had an increased risk of death and relapse compared with patients with low P4HB and HIF-1α expression after adjusting for potential confounders. Based on our research, we suggest that higher expression of P4HB and HIF-1α promotes risk of death and relapse and may act as an indicator of prognosis in patients with gastric cancer.
- Citation: Zhang J, Wu Y, Lin YH, Guo S, Ning PF, Zheng ZC, Wang Y, Zhao Y. Prognostic value of hypoxia-inducible factor-1 alpha and prolyl 4-hydroxylase beta polypeptide overexpression in gastric cancer. World J Gastroenterol 2018; 24(22): 2381-2391
- URL: https://www.wjgnet.com/1007-9327/full/v24/i22/2381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i22.2381
Despite a decline in the incidence of gastric cancer (GC) globally in recent years[1], this form of cancer is still the second most common type of cancer and the second leading cause of cancer-related death in China[2]. This is attributed to its specific biological characteristics: a lack of early clinical manifestation, local invasion, and systemic metastasis. Most patients with GC are diagnosed at an advanced stage. Although traditional treatment strategies have improved, the median overall survival (OS) of advanced GC is less than 1 year[3], with more than half of patients having a recurrence within 5 years of surgery and adjuvant chemotherapy[1]. As a result, it is urgent to identify effective biomarkers to aid in prognostic accuracy as a part of therapy against GC.
Considerable evidence has shown that the hypoxic tumor microenvironment is closely associated with cancer progression[4] and metastasis[5]. Hypoxia-inducible factors (HIFs), HIF-1 and HIF-2, play a co-operative role in mediating the cellular response to low oxygen tension[6]. In response to hypoxia, the expression of these factors are self-regulated, resulting in the acceleration of cell dissemination, invasion, and angiogenic properties, as well as a shift among cancer cells towards a metastatic phenotype[5]. Specifically, HIF-1α, a subunit of HIF-1, has been shown to upregulate epithelial mesenchymal transition-related transcription factors (EMT-TFs)[7], and to antagonize p53[8].
In the last decade, a plethora of targeted molecular therapies have been approved and used in clinical practice based on gene expression profiling research. However, the efficacy of these drugs has not met expectations, and the anticipated breakthroughs in treatment resulting from their use have not been achieved[9]. As tumor invasion and metastasis requires multiple molecular alterations, a reasonable combination of several biomarkers may improve prognostic accuracy.
In previous research, we identified prolyl 4-hydroxylase beta polypeptide (P4HB) as a putative downstream molecular target of HIF-1α based on bioinformatics prediction and experimentation. These molecules affected the invasion and metastasis of GC. P4HB is an abundant multifunctional enzyme that belongs to the protein disulfide isomerase (PDI) family[10]. It can act as an endoplasmic reticulum (ER) chaperone to inhibit the aggregation of misfolded proteins[11]. Studies have shown that P4HB is overexpressed in hepatocellular carcinoma[12,13] and has a close relationship with drug resistance in malignant glioma[14] and non-small cell lung cancer[15].
The aim of this study was to investigate the correlation between HIF-1α, P4HB expression, and relevant clinicopathologic parameters in GC patients. Furthermore, we also evaluated whether prognosis and hepatic/peritoneum metastasis are associated with HIF-1α or P4HB expression.
This study was conducted with approval of the Liaoning Province Cancer Hospital and Institute Institutional Review Board. Informed consent for specimen preservation was obtained from patients before their surgery at Liaoning Province Cancer Hospital and Institute.
This retrospective study enrolled a total of 428 patients with GC who underwent gastrectomy between January 2009 and December 2011 at Liaoning Province Cancer Hospital & Institute (Liaoning, China). All patients underwent initial curative gastrectomy, 423 cases (98.8%) were D2 lymph node dissected, and five cases (1.2%) were D1 lymph node dissected. None of the enrolled patients received chemotherapy or radiotherapy before surgery. All enrolled patients had a sufficient number of paraffin embedded tumor specimens stored. Adenosquamous carcinoma or neuroendocrine carcinoma patients were excluded from our analysis. Tumor staging conformed to the eighth edition of the American Joint Committee on Cancer/Union International Control Center (AJCC/UICC) TNM staging manual (2017). The median patient age at surgery was 58 years (range 32-81 years), 304 (71.0%) were male, and 124 (29.0%) were female. All patients with staging more advanced than IIA received adjuvant chemotherapy. The clinicopathological parameters of patients included age, gender, Borrmann type, tumor size, tumor histological morphology, Lauren type, tumor differentiation, T category, N category, TNM stage, vascular invasion, perineural invasion, and operation (Table 1).
| Characteristics | n (%) | DFS | OS | ||||
| t/mo | P value | χ2/Z | t/mo | P value | χ2/Z | ||
| Age (median, yr) | 0.47 | -0.72 | 0.79 | -0.26 | |||
| < 60 | 199 (46.5) | 40.02 | 44.97 | ||||
| ≥ 60 | 229 (53.5) | 37.72 | 44.34 | ||||
| Gender | 0.42 | -0.80 | 0.56 | -0.59 | |||
| Male | 304 (71.0) | 37.74 | 43.99 | ||||
| Female | 124 (29.0) | 41.38 | 46.23 | ||||
| Bormann type | 0.00 | 78.58 | 0.00 | 70.97 | |||
| I | 33 (7.7) | 64.24 | 64.21 | ||||
| II | 189 (44.2) | 45.99 | 51.15 | ||||
| III | 200 (46.7) | 28.43 | 35.78 | ||||
| IV | 6 (1.4) | 17.50 | 27.33 | ||||
| Tumor size | 0.00 | -6.17 | 0.00 | -5.87 | |||
| < 5 cm | 243 (56.8) | 46.15 | 50.57 | ||||
| ≥ 5 cm | 185 (43.2) | 29.12 | 36.84 | ||||
| Location | 0.09 | 4.92 | 0.22 | 3.03 | |||
| Up | 90 (21.0) | 33.10 | 40.62 | ||||
| Middle | 121 (28.3) | 39.98 | 45.26 | ||||
| Low | 217 (50.7) | 40.49 | 45.96 | ||||
| Tumor histological morphology | 0.26 | 2.70 | 0.36 | 2.03 | |||
| Adenocarcinoma | 319 (74.5) | 39.45 | 45.15 | ||||
| Absolute signet ring cell carcinoma | 3 (0.7) | 63.67 | 63.67 | ||||
| Mixed carcinoma | 106 (24.8) | 36.09 | 42.56 | ||||
| Lauren type | 0.07 | 5.28 | 0.06 | 5.50 | |||
| Intestinal | 200 (49.5) | 41.74 | 47.59 | ||||
| Diffuse | 129 (26.9) | 39.28 | 44.97 | ||||
| Mixed type | 99 (23.1) | 33.96 | 40.12 | ||||
| Tumor differentiation | 0.01 | -2.74 | 0.01 | -2.54 | |||
| Poor | 288 (67.3) | 36.54 | 42.72 | ||||
| Moderate and high | 140 (32.7) | 43.43 | 48.58 | ||||
| Vessel invasion | 0.00 | -6.06 | 0.00 | -5.65 | |||
| No | 299 (69.9) | 43.24 | 48.60 | ||||
| Yes | 129 (30.1) | 28.49 | 35.47 | ||||
| Perineural invasion | 0.00 | -5.63 | 0.00 | -5.26 | |||
| No | 320 (74.8) | 42.76 | 47.95 | ||||
| Yes | 108 (25.2) | 27.03 | 34.82 | ||||
| T category | 0.00 | 143.15 | 0.00 | 124.25 | |||
| T1 | 85 (19.9) | 65.15 | 65.20 | ||||
| T2 | 24 (5.6) | 61.13 | 62.44 | ||||
| T3 | 16 (3.7) | 59.04 | 61.33 | ||||
| T4 | 303 (70.8) | 28.61 | 36.61 | ||||
| N category | 0.00 | 323.31 | 0.00 | 322.11 | |||
| N0 | 179 (41.8) | 62.20 | 63.47 | ||||
| N1 | 67 (15.7) | 37.22 | 48.52 | ||||
| N2 | 84 (19.6) | 23.48 | 33.05 | ||||
| N3 | 98 (22.9) | 10.24 | 17.52 | ||||
| TNM stage | 0.00 | 285.22 | 0.00 | 271.25 | |||
| I | 93 (21.7) | 64.16 | 64.20 | ||||
| II | 101 (23.6) | 63.27 | 65.59 | ||||
| III | 228 (53.3) | 18.44 | 28.30 | ||||
| IV | 6 (1.4) | 7.00 | 9.50 | ||||
| Operation | 0.00 | -3.45 | 0.00 | -3.79 | |||
| D1 | 5 (1.2) | 7.00 | 9.20 | ||||
| D2 | 423 (98.8) | 39.17 | 45.06 | ||||
| Hepatic metastasis | 0.00 | -5.31 | 0.00 | -5.10 | |||
| Yes | 46 (10.7) | 18.48 | 28.22 | ||||
| No | 382 (89.3) | 41.24 | 46.62 | ||||
| Peritoneum cavity metastasis | 0.00 | -14.35 | 0.00 | -13.96 | |||
| Yes | 155 (36.2) | 14.45 | 23.47 | ||||
| No | 273 (63.8) | 52.61 | 56.66 | ||||
| HIF-1α | 0.00 | -4.41 | 0.00 | -4.24 | |||
| Weak-expression | 209 (48.8) | 44.80 | 49.58 | ||||
| Over- expression | 219 (51.2) | 33.06 | 39.92 | ||||
| P4HB | 0.00 | -6.88 | 0.00 | -6.91 | |||
| Weak-expression | 213 (49.8) | 48.03 | 52.48 | ||||
| Over- expression | 215 (50.2) | 29.64 | 36.87 | ||||
Follow-up assessment included physical examination, complete blood count, liver function test, endoscope, pulmonary, abdominal, and pelvic computed tomography (CT) scan. Follow-up assessments were performed every 3-6 mo for 5 years after surgery. The date of first relapse and date of death were recorded in applicable cases. Survival was calculated from the time of surgery until the last follow-up or death from any cause. The last follow-up data included were from July 1, 2017. The first relapse date and death date were recorded. Disease-free survival (DFS) was defined as the period between surgery and the first relapse diagnosis. Overall survival (OS) was defined as the period between the resection date and death for any cause.
Serial sections (5 μm) of tumor tissue and adjacent tissue were fixed on poly-L-lysine-coated slides. Each section was immunostained with antibodies against HIF-1α (Abcam, 1:100) and P4HB (BOSTER, 1:50) and visualized by incubation with the appropriate biotin-conjugated secondary antibodies and immmunoperoxidase detection using the VECTASTAIN ABC Elite kit (Linaris, Werthein, Germany) and diaminobenzidine (DAB) substrate (BOSTER, China).
Immunoreactivity of HIF-1α and P4HB was evaluated independently by two experienced pathologists. The final results of the evaluation were obtained after considering the staining intensity of the proportion of positive cells. This proportion was calculated as the mean percentage of gene-positive tumor cells elevated in five areas at 200 × magnification. The proportion of positive cells was considered from the following: 0, negative; 1, positive in ≤ 10% of cells; 2, positive in > 10% and ≤ 50% of cells; 3, positive in > 50% and ≤ 75% of cells; and 4, positive in > 75% of cells. Then, staining intensity of this level was scored as 0, negative; 1, weak; 2, moderate; and 3, strong. The two scores were multiplied and expressed as a graded: 0, negative; 1-4, weak expression; 5-8, moderate expression; and 9-12, strong expression. The median score was used as the expression cutoff point (HIF-1α median score = 6, P4HB median score = 4). Patients were divided into over-expression or weak-expression groups based on these values.
Genes related to HIF-1α and P4HB
We used the online database Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancerpku.cn/index.html)[16], an interactive web server, to analyze our RNA sequencing expression data based on The Cancer Genome Atlas (TCGA) and the Genotype-Tissue Expression (GTEx) projects.
Statistical Package for Social Science (SPSS), version 19.0 (Armonk, NY, United States) was used for statistical analysis. The χ2 test or Fisher’s Exact test was used to explore the correlations among clinicopathological variables and biomarker variables (HIF-1α and P4HB). Survival curves were analyzed by the Kaplan-Meier method, and univariate survival analysis was performed using log-rank test. Cox proportional hazards model was used to examine the effects on survival for prognostic biomarkers.
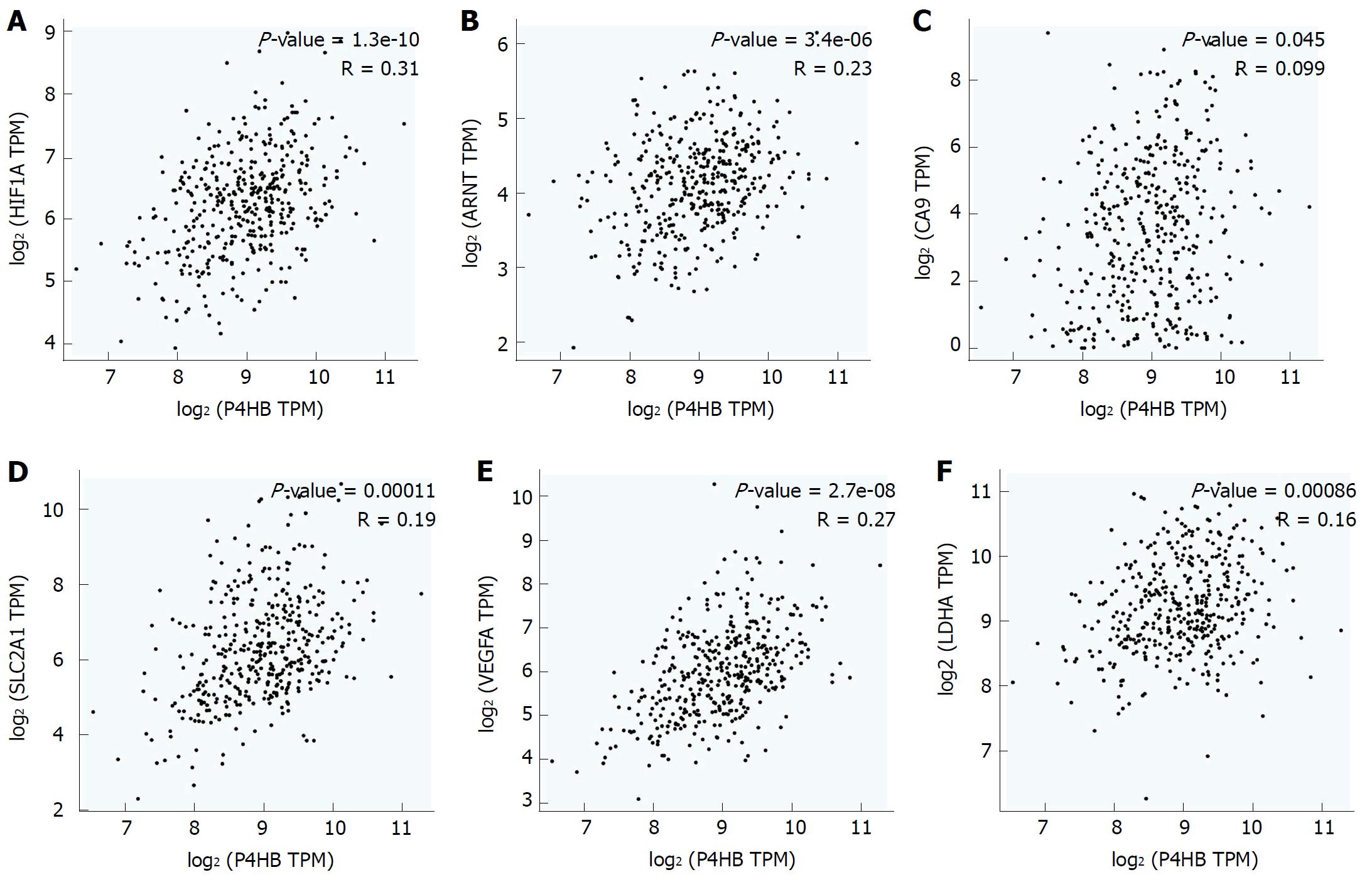
In previous research, we demonstrated that P4HB is downstream of HIF-1α. Inhibition of HIF-1α expression down-regulates the expression of P4HB. Therefore, we suspected P4HB is co-expressed with other hypoxia-associated genes. To examine this hypothesis, we explored the correlation between P4HB and HIF-1α and hypoxia-associate biomarkers: hydrocarbon receptor nuclear translocator (ARNT), carbonic anhydrase 9 (CA9), egl-9 aryl solute carrier family 2 member 1 (SLC2A1 or Glut-1), lactate dehydrogenase A (LDHA or LDH5), and vascular endothelial growth factor A (VEGFA) in GEPIA. Higher P4HB expression was observed in our database with high HIF-1α (R = 0.31, P = 1.3e-10), ARNT (R = 0.23, P = 3.4e-6), CA9 (R = 0.099, P = 0.045), SLC2A1 (R = 0.19, P = 0.00011), VEGFA (R = 0.27, P = 2.7e-8), and LDHA (R = 0.16, P = 0.00086) (Figure 1A-F).
HIF-1α and P4HB expression in GC specimens
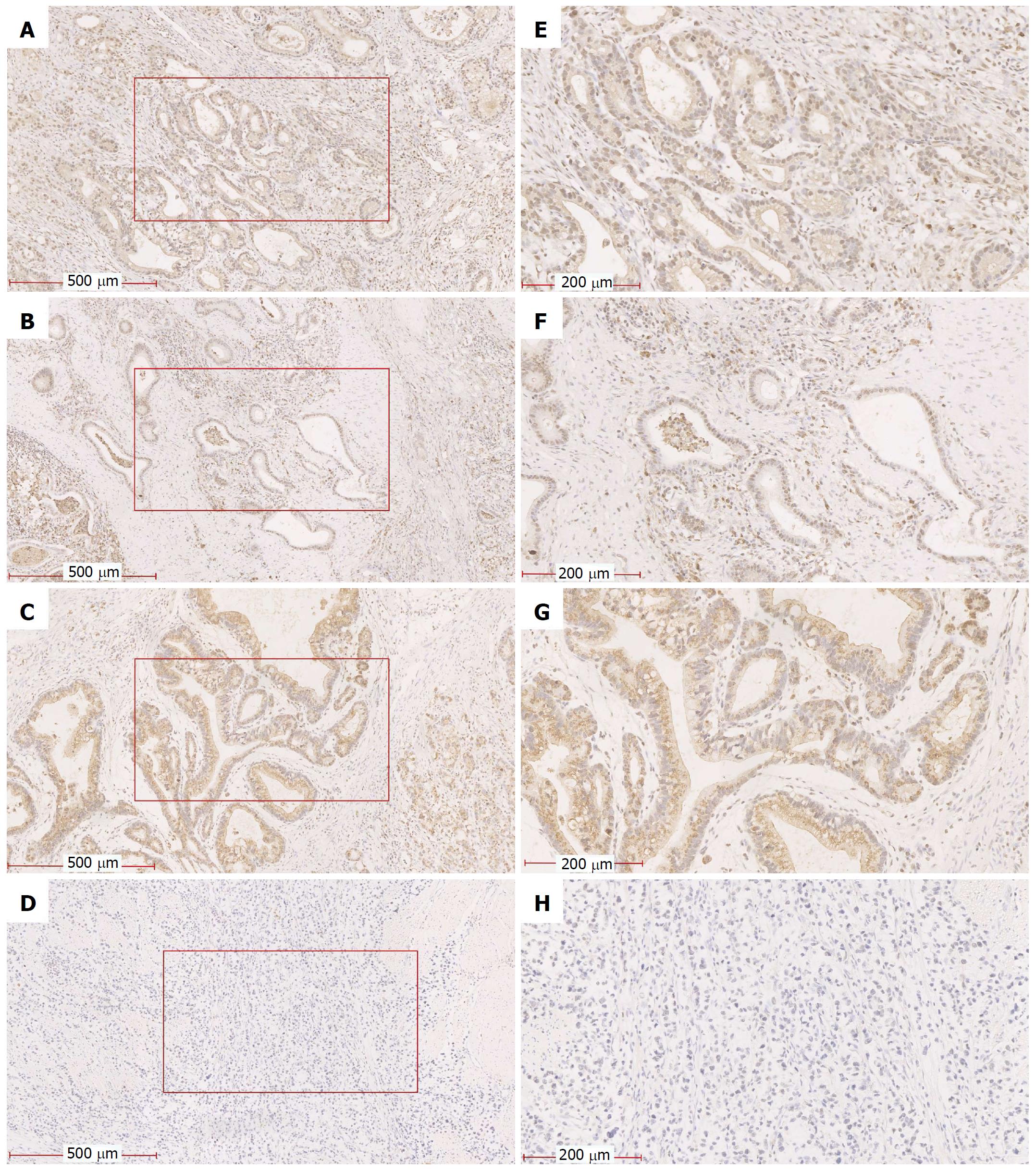
To investigate the role of HIF-1α and P4HB in GC tissue, we detected the expression of HIF-1α and P4HB by immunohistochemistry (IHC) in a total of 428 GC samples tissues. We found that 51.2% (n = 219) of tissues showed HIF-1α with strongly positive expression (Figure 2A), and 44.8% (n = 209) showed weakly positive expression (Figure 2B). Additionally, 50.2% (n = 215) of tissues showed P4HB with strongly positive expression (Figure 2C), and 49.8% (n = 213) showed weakly positive expression (Figure 2D). The number of patients with both genes over-expressed was 174, and it was 168 for weak-expression. The number of patients only with HIF-1α over-expression was 45 and with only P4HB over-expression was 41.
Correlation between the expression of HIF-1α, P4HB, and clinicopathologic factors
The association between clinicopathological characteristics and the expression of HIF-1α and P4HB are summarized in Table 2. According to χ2 test or Fisher’s Exact test, HIF-1α overexpression was associated with T category (P = 0.01, χ2 = 10.93), N category (P = 0.00, χ2 = 23.55), TNM stage (P = 0.00, χ2 = 23.32), hepatic metastases (P = 0.01, χ2 = 6.98), and peritoneum cavity metastasis (P = 0.00, χ2 = 12.67). P4HB overexpression was associated with Bormann type (P = 0.00, χ2 = 29.18), tumor size (P = 0.00, χ2 = 11.09), tumor differentiation (P = 0.03, χ2 = 4.53), perineural invasion (P = 0.03, χ2 = 4.71), T category (P = 0.00, χ2 = 44.94), N category (P = 0.00, χ2 = 54.13), TNM stage (P = 0.00, χ2 = 65.64), and peritoneum cavity metastasis (P = 0.00, χ2 = 39.29).
| Characteristics | HIF-1α | P4HB | ||||||
| Low | High | P value | χ2 | Low | High | P value | χ2 | |
| 209 (48.8) | 219 (51.2) | 213 (49.8) | 215 (50.2) | |||||
| Age | 0.42 | 0.65 | 0.35 | 0.57 | ||||
| < 60 | 93 (46.7) | 106 (53.3) | 96 (48.2) | 103 (51.8) | ||||
| ≥ 60 | 116 (50.7) | 113 (49.3) | 117 (51.1) | 112 (48.9) | ||||
| Gender | 0.91 | 0.01 | 0.48 | 0.49 | ||||
| Male | 149 (49.0) | 155 (51.0) | 148 (48.7) | 156 (51.3) | ||||
| Female | 60 (48.4) | 64 (51.6) | 65 (52.4) | 59 (47.6) | ||||
| Bormann type | 0.06 | 7.26 | 0.00 | 29.18 | ||||
| I | 16 (48.5) | 17 (51.5) | 26 (78.8) | 7 (21.2) | ||||
| II | 104 (55.0) | 85 (45.0) | 110 (58.2) | 79 (41.8) | ||||
| III | 88 (44.0) | 112 (56.0) | 75 (37.5) | 125 (62.5) | ||||
| V | 1 (16.7) | 5 (83.3) | 2 (33.3) | 4 (66.7) | ||||
| Tumor size | 0.30 | 1.09 | 0.00 | 11.09 | ||||
| < 5 cm | 124 (51.0) | 119 (49.0) | 138 (42.0) | 110 (58.0) | ||||
| ≥ 5 cm | 85 (45.9) | 100 (54.1) | 75 (14.6) | 105 (85.4) | ||||
| Tumor histological morphology | 0.18 | 3.45 | 0.49 | 1.42 | ||||
| Adenocarcinoma | 157 (49.2) | 162 (50.8) | 163 (51.1) | 156 (48.9) | ||||
| Absolute signet ring cell carcinoma | 3 (100.0) | 0 (0.0) | 2 (66.7) | 1 (33.3) | ||||
| Mixed carcinoma | 49 (46.2) | 57 (53.8) | 48 (45.3) | 58 (54.7) | ||||
| Location | 0.15 | 3.78 | 0.63 | 0.94 | ||||
| Up | 40 (14.4) | 50 (55.6) | 41 (45.6) | 49 (54.4) | ||||
| Middle | 53 (43.8) | 68 (56.2) | 60 (49.6) | 61 (50.4) | ||||
| Low | 116 (53.5) | 101 (46.5) | 112 (51.6) | 105 (48.4) | ||||
| Lauren type | 0.05 | 5.98 | 0.57 | 1.14 | ||||
| Intestinal | 96 (48.0) | 104 (52.0) | 104 (52.0) | 96 (48.0) | ||||
| Diffuse | 73 (56.6) | 56 (43.4) | 64 (49.6) | 65 (50.4) | ||||
| Mixed type | 40 (40.4) | 59 (59.6) | 45 (45.5) | 54 (54.5) | ||||
| Tumor differentiation | 0.08 | 3.17 | 0.03 | 4.53 | ||||
| Poor | 132 (45.8) | 156 (54.2) | 133 (46.2) | 155 (53.8) | ||||
| Moderate and high | 77 (55.0) | 63 (45.0) | 80 (57.1) | 60 (42.9) | ||||
| Vessel invasion | 0.21 | 1.60 | 0.38 | 0.78 | ||||
| No | 152 (50.8) | 147 (49.2) | 153 (51.2) | 146 (48.8) | ||||
| Yes | 57 (44.2) | 72 (55.8) | 60 (46.5) | 69 (53.5) | ||||
| Perineural invasion | 0.41 | 0.69 | 0.03 | 4.71 | ||||
| No | 160 (50.0) | 160 (50.0) | 169 (52.8) | 151 (47.2) | ||||
| Yes | 49 (45.4) | 59 (54.6) | 44 (40.7) | 92 (59.3) | ||||
| T category | 0.01 | 10.93 | 0.00 | 44.94 | ||||
| T1 | 32 (88.9) | 4 (11.1) | 9 (56.3) | 7 (43.8) | ||||
| T2 | 11 (45.8) | 13 (54.2) | 13 (54.2) | 11 (45.8) | ||||
| T3 | 41 (45.1) | 50 (54.9) | 69 (81.2) | 16 (18.8) | ||||
| T4 | 125 (45.1) | 152 (54.9) | 122 (40.3) | 181 (59.7) | ||||
| N category | 0.00 | 23.55 | 0.00 | 54.13 | ||||
| N0 | 111 (62.0) | 68 (38.0) | 126 (70.4) | 53 (29.6) | ||||
| N1 | 28 (41.8) | 39 (58.2) | 28 (41.8) | 39 (58.2) | ||||
| N2 | 37 (44.0) | 47 (56.0 | 28 (33.3) | 56 (66.7) | ||||
| N3 | 33 (33.7) | 65 (66.3) | 31 (31.6) | 67 (68.4) | ||||
| TNM stage | 0.00 | 23.32 | 0.00 | 65.64 | ||||
| I | 54 (58.1) | 39 (41.9) | 72 (77.4) | 21 (22.6) | ||||
| II | 65 (64.4) | 36 (35.6) | 65 (64.4) | 36 (35.6) | ||||
| III | 87 (38.2) | 141 (61.8) | 75 (32.9) | 1503 (67.1) | ||||
| IV | 3 (50.0) | 3 (50.0) | 1 (16.7) | 5 (83.3) | ||||
| Operation | 0.69 | 0.16 | 0.18 | 1.79 | ||||
| D1 | 2 (40.0) | 3 (60.0) | 1 (20.0) | 4 (80.0) | ||||
| D2 | 207 (48.9) | 216 (51.1) | 212 (50.1) | 211 (49.9) | ||||
| Hepatic metastases | 0.01 | 6.98 | 0.07 | 3.38 | ||||
| Yes | 14 (30.4) | 32 (69.6) | 17 (37.0) | 29 (63.0) | ||||
| No | 195 (51.0) | 187 (49.0) | 196 (51.3) | 186 (48.7) | ||||
| Peritoneum cavity metastasis | 0.00 | 12.67 | 0.00 | 39.29 | ||||
| Yes | 58 (37.4) | 97 (62.6) | 46 (29.7) | 109 (70.3) | ||||
| No | 151 (55.3) | 122 (44.7) | 167 (61.2) | 106 (38.8) | ||||
In this series, 416 of 428 patients had available clinical follow-up data. Specifically, 250 patients (58.4%) died prior to end of follow-up. Of these, 155 (36.2%) and 46 (10.7%) developed peritoneum cavity metastasis and hepatic metastasis, respectively. The median DFS was 31.50 mo (range 5-91), and the median OS was 45.00 mo (range 7-91).
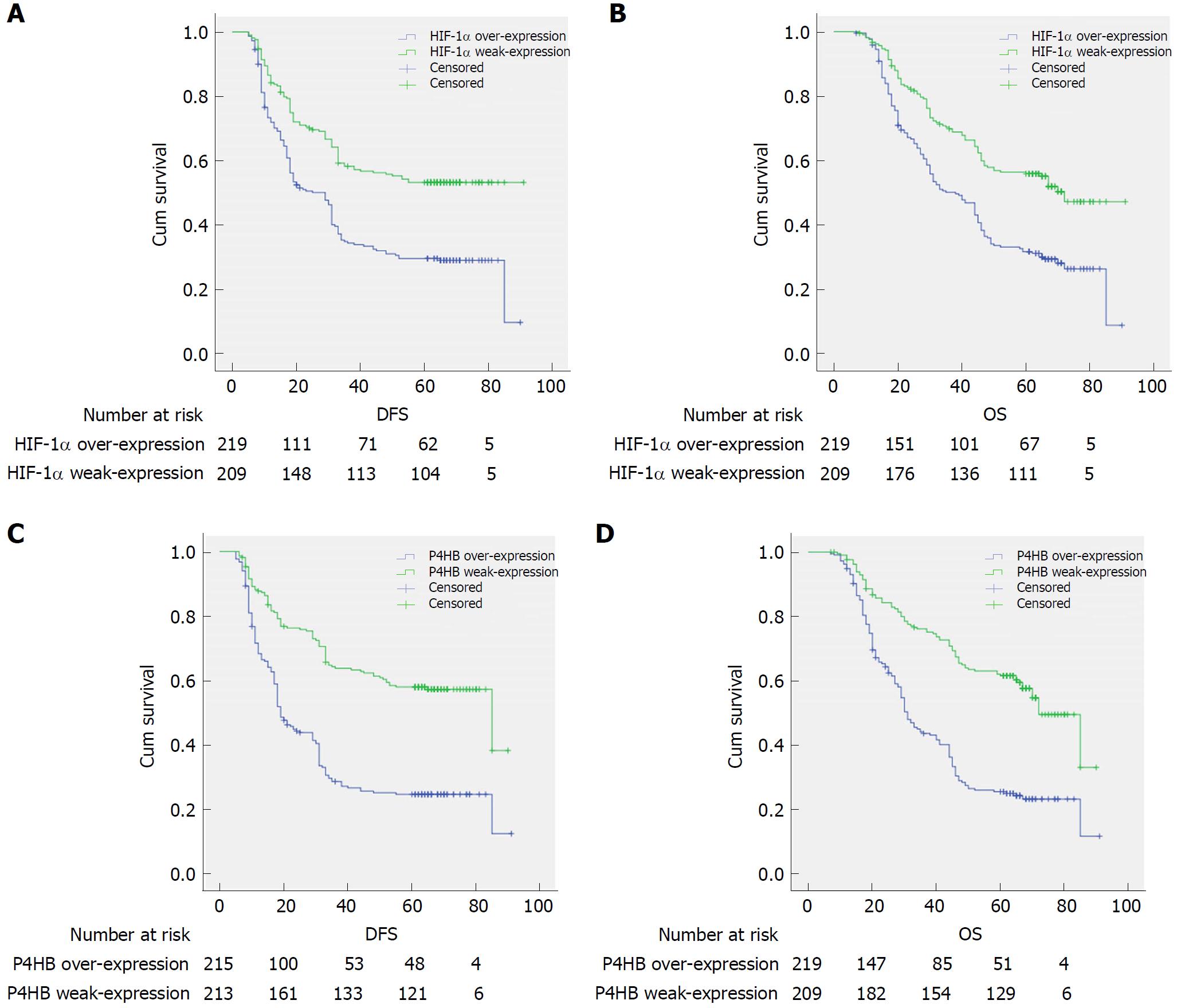
Clinical outcomes and clinicopathological factors were evaluated by Kaplan-Merier method and log-rank test. These global prognostic factors were undoubtedly associated with DFS and OS, such as: Bormann type, tumor size, tumor differentiation, vessel invasion, perineural invasion, T category, N category, TNM stage, operation, hepatic metastasis, and peritoneum cavity metastasis. HIF-1α-weak-expression patients displayed a longer DFS (44.80 mo vs 22.06 mo) and OS (49.58 mo vs 39.92 mo) (Table 1, Figure 3A and C). Patients with strong-expression of P4HB had a shorter survival time than those with weak-expression (DFS: 48.03 mo vs 29.64 mo, OS: 52.48 mo vs 36.87 mo, Table 1, Figure 3B and D).
Multivariate analysis used factors with P < 0.05 in univariate analysis. A backward stepwise method was used in the Cox proportional hazards model. After adjusting for Bormann type, tumor size, TNM stage, and other variables, TNM stage was undoubtedly associated with shorter DFS and OS (DFS: P = 0.00, HR = 24.65, 95%CI: 16.04-37.87; OS: P = 0.00, HR = 87.72, 95%CI: 41.21-186.68). Vessel invasion amplification was associated with shorter DFS (DFS: P = 0.00, HR = 0.70, 95%CI: 0.53-0.93). Finally HIF-1α over-expression was an independent prognostic factor predicting DFS and OS (DFS: P = 0.00, HR = 0.67, 95%CI: 0.52-0.88, OS: P = 0.00, HR = 0.65, 95%CI: 0.50-0.85). However, P4HB was only the independent prognostic factor predicting DFS but not OS (DFS: P = 0.04, HR = 0.76, 95%CI: 0.58-1.00, OS: P = 0.65, HR = 0.94, 95%CI: 0.72-1.23) (Table 3).
| Variables | DFS | OS | ||||
| P value | HR | 95%CI | P value | HR | 95%CI | |
| Bormann | 0.26 | 1.15 | 0.91-1.46 | 0.38 | 1.11 | 0.88-1.40 |
| Tumor Size | 0.34 | 0.88 | 0.67-1.15 | 0.38 | 0.89 | 0.68-1.16 |
| Tumor differentiation | 0.95 | 0.99 | 0.74-1.33 | 0.84 | 1.03 | 0.77-1.31 |
| Vessel invasion | 0.01 | 0.70 | 0.53-0.93 | 0.19 | 0.83 | 0.63-1.09 |
| Perineural invasion | 0.44 | 0.90 | 0.68-1.19 | 0.25 | 0.86 | 0.66-1.11 |
| TNM stage | 0.00 | 24.65 | 16.04-37.87 | 0.00 | 87.72 | 41.21-186.68 |
| HIF-1α expression | 0.00 | 0.67 | 0.52-0.88 | 0.00 | 0.65 | 0.50-0.85 |
| P4HB expression | 0.04 | 0.76 | 0.58-1.00 | 0.65 | 0.94 | 0.72-1.23 |
Metastasis is the most common cause of treatment failure in cancer, and its process is complex and highly orchestrated. Studies have demonstrated that not only the primary tumor but also the distant tissue microenvironment influence the propensity for tumor metastasis[17,18]. Oxygen is an essential nutrient in normal tissue as well as in facilitating tumor spread. When solid tumors grow faster than oxygen delivery, they use the hypoxic signaling pathway to maintain their oxygen supply and adapt to hypoxic conditions[19]. Regardless of the initiation site, tumor metastasis is promoted by HIF signaling; specifically, HIF-1 and HIF-2 coordinate to stabilize this pathway and maintain homeostasis of the hypoxic microenvironment[20]. As a key transcription factor, HIF-1α is involved in each step of the tumor metastatic cascade: from epithelial-mesenchymal transition (EMT)[7], intravasation[21], extravasation[22], the establishment of premetastatic niche to survival at a distant site[23]. In vitro and in vivo experiments have shown that downregulation of HIF-1α expression can significantly inhibit the malignancy phenotype of GC[24,25]. However, as a malignant tumor with significant heterogeneity, the prognostic role of HIF-1α in GC is remains controversial.
Four systematic reviews (Lin et al[26], Chen et al[27], Zhu et al[28], Zhang et al[29]) have reported on the prognostic role of HIF-1α in GC. Briefly, all of the meta-analyses consider HIF-1α to have a remarkable correlation with poor OS in GC, but whether HIF-1α has a close relationship with DFS or PFS is still unclarified. Meanwhile, a limitation in these meta-analyses is sample size, as the largest sample size involved in the studies was 216. In comparison, we collected samples from 428 patients in our research. The univariate and multivariate analysis highlighted the overexpression of HIF-1α as an independent factor in predicting DFS and OS of GC; this conclusion is similar to that of Li et al[30].
In a previous study, we demonstrated that P4HB is a potential target of HIF-1α with bioinformatics analysis and molecular biology. P4HB functions primarily as the beta subunit of prolyl 4-hydroxylase, forming a tetrameric enzyme with P4HA1 or P4HA2 subunits. Although the mechanism of interaction between HIF-1α and P4HB is not clear, some studies suggest that HIF-1 promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1[31] and P4HA2 in breast cancer[32]. Moreover, P4HA1[33] and P4HA2[34] are also treated as a hypoxia-associated gene in head and neck squamous cell carcinoma (HNSCC), and its signature is a prognostic tool in treatment of HNSCC patients. The interaction between P4HA1/2 has also been reported in chondrosarcoma cells[35] and soft tissue sarcomas[36], but whether P4HA1/2 is directly regulated by HIF-1 has not been reported.
It is possible that P4HB acts as a bridge between HIF-1α and P4HA1/2. P4HB, also named PDIA1, is a promising chemotherapy target in ovarian cancer cells[37] and acts as an oncogene in melanoma[38]. PDIA6 is an upstream gene of the Wnt/β-catenin signaling pathway affected by the proliferation and growth of bladder cancer cells[39]. ERp19 knockdown of GC cells dramatically suppressed cell growth and inhibited cellular migration, whereas ERp19 over-expression reversed these changes[40]. In light of these findings, PDI proteins are viewed as prognostic factors for clinical use, but the molecular regulation mechanisms of P4HB in GC is unclear. Hence, our present study confirmed P4HB as an independent prognostic factor in GC patient DFS for the first time.
We analyzed the relationship between HIF-1α and P4HB expression as well as with clinicopathologic characteristics. This analysis reveals that protein overexpression was correlated with both TNM stage and peritoneum cavity metastasis. However, it is highly likely that TNM stage is a crucial prognostic factor. Peritoneum cavity metastasis is the most frequent pattern seen in postoperative recurrence in GC. Together, they can determine the prognosis of GC patients.
The positive correlation between biomarker protein expression and the decisive factors (TNM stages and peritoneum cavity metastasis) implies an assignable effect of HIF-1α and P4HB in prognosis of GC. In reviewing the entire dataset, we found that HIF-1α may be more meaningful than P4HB to clinical data: HIF-1α is associated with DFS and OS, but P4HB is only meaningful to DFS. This also indirectly proves that HIF-1α, as the upstream gene, is involved in more signaling pathways than P4HB, which may be related to recurrence. Thus, the effect of HIF-1α is broad, not only for cancer, but also in the development of normal tissue. This suggests potential dire consequences for complete targeted HIF-1α silencing at the genetic level. Therefore, it is very important to clarify the precise regulation relationship of HIF-1α and to identify the specific downstream target genes.
Although we have demonstrated the significance of HIF-1α and P4HB in GC prognosis, the limitations of this study, such as its retrospective study approach and restricted sample size, should not be neglected. Furthermore, we did not include analysis of neoadjuvant chemotherapy as a factor affecting patient outcomes in this study. Further studies should be undertaken to understand better the molecular mechanism between other biomarkers and survival in GC.
The poor prognosis of patients diagnosed with gastric cancer (GC) reflects the limitation of our arsenal of anticancer therapeutic strategies. Hypoxia is a critical factor that shapes the GC microenvironment. However, the involvement of GC cells under such conditions remains poorly explained. Although the core transcription factor of the hypoxia signal pathway hypoxia-inducible factor-1α (HIF-1α) has been studied for decades, its prognostic value in GC is still unclear and controversial.
In a previous report, we demonstrated that prolyl 4-hydroxylase beta (P4HB) is a potential target of HIF-1α, but the exact mechanism was not determined.
The aim of the present study is to evaluate the prognostic value of HIF-1α and P4HB in GC.
This study included 428 patients with confirmed GC who underwent gastrectomy in a single Chinese Cancer Center between 2009 and 2011. Clinicopathologic features as well as immunohistochemical analysis of HIF- 1a and P4HB were determined. Long-term survival of these patients was analyzed using univariate and multivariate analyses.
P4HB was positively correlated with hypoxia-associated genes. HIF-1α and P4HB overexpression were significantly correlation with TNM staging and peritoneum cavity metastasis. In univariate analysis, patients with high HIF-1α expression trend had a shorter disease-free survival and overall survival. P4HB overexpression exhibited similar results. Furthermore, HIF-1α is a clinicopathological predictor of dismal prognosis by multivariate analysis.
The prognostic value of P4HB in GC was first reported. We confirmed that HIF-1α overexpression could be considered a useful independent prognostic biomarker in GC after gastrectomy and is correlated to both poor overall survival and disease-free survival. Taken together with our previous research, we are more determined that P4HB is a hypoxia-associated gene and regulated by HIF-1α.
Our research team will explore the mutual regulatory mechanism of HIF-1 and P4HB in future studies through mass spectrometry analysis and co-immunoprecipitation. In addition, we will explore the relationship between the two biomarkers by establishing animal models.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Barreto S, Kanat O, Kosugi A, Tanabe S S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12176] [Article Influence: 1522.0] [Reference Citation Analysis (3)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13189] [Article Influence: 1465.4] [Reference Citation Analysis (3)] |
| 3. | Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, Bang YJ, Alcindor T, O’Callaghan CJ, Burnell MJ. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol. 2016;34:2728-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, Saltz L, Paty PB, Tavazoie SF. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160:393-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 292] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 5. | Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 978] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 6. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4392] [Cited by in RCA: 4713] [Article Influence: 157.1] [Reference Citation Analysis (0)] |
| 7. | Tsai YP, Chen HF, Chen SY, Cheng WC, Wang HW, Shen ZJ, Song C, Teng SC, He C, Wu KJ. TET1 regulates hypoxia-induced epithelial-mesenchymal transition by acting as a co-activator. Genome Biol. 2014;15:513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Amelio I, Melino G. The p53 family and the hypoxia-inducible factors (HIFs): determinants of cancer progression. Trends Biochem Sci. 2015;40:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much? Clin Cancer Res. 2010;16:5972-5980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Chen GQ, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565-6578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 835] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 11. | Noiva R. Protein disulfide isomerase: the multifunctional redox chaperone of the endoplasmic reticulum. Semin Cell Dev Biol. 1999;10:481-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 207] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Lin H, Yan J, Wang Z, Hua F, Yu J, Sun W, Li K, Liu H, Yang H, Lv Q. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology. 2013;57:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Xia W, Zhuang J, Wang G, Ni J, Wang J, Ye Y. P4HB promotes HCC tumorigenesis through downregulation of GRP78 and subsequent upregulation of epithelial-to-mesenchymal transition. Oncotarget. 2017;8:8512-8521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Sun S, Wong TS, Zhang XQ, Pu JK, Lee NP, Day PJ, Ng GK, Lui WM, Leung GK. Protein alterations associated with temozolomide resistance in subclones of human glioblastoma cell lines. J Neurooncol. 2012;107:89-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Wang SM, Lin LZ, Zhou DH, Zhou JX, Xiong SQ. Expression of prolyl 4-hydroxylase beta-polypeptide in non-small cell lung cancer treated with Chinese medicines. Chin J Integr Med. 2015;21:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98-W102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5550] [Cited by in RCA: 7022] [Article Influence: 877.8] [Reference Citation Analysis (0)] |
| 17. | Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol Sci. 2016;37:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 18. | Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1012] [Cited by in RCA: 946] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 19. | Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1304] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 20. | Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2389] [Cited by in RCA: 2482] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 21. | Deryugina EI, Kiosses WB. Intratumoral Cancer Cell Intravasation Can Occur Independent of Invasion into the Adjacent Stroma. Cell Rep. 2017;19:601-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Hunfeld A, Segelcke D, Bäcker I, Mecheri B, Hemmer K, Dlugosch E, Andriske M, Paris F, Zhu X, Lübbert H. Hypoxia facilitates neurogenic dural plasma protein extravasation in mice: a novel animal model for migraine pathophysiology. Sci Rep. 2015;5:17845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic Effects of p53 and HIF1A on microRNA-34a Regulation of PPP1R11 and STAT3 and Hypoxia-induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells. Gastroenterology. 2017;153:505-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Seok JK, Lee SH, Kim MJ, Lee YM. MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014;42:8062-8072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Gan L, Meng J, Xu M, Liu M, Qi Y, Tan C, Wang Y, Zhang P, Weng W, Sheng W. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin β4/FAK/SOX2/HIF-1α signaling pathway in gastric cancer. Oncogene. 2018;37:744-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Lin S, Ma R, Zheng XY, Yu H, Liang X, Lin H, Cai XJ. Meta-analysis of immunohistochemical expression of hypoxia inducible factor-1α as a prognostic role in gastric cancer. World J Gastroenterol. 2014;20:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Chen J, Li T, Liu Q, Jiao H, Yang W, Liu X, Huo Z. Clinical and prognostic significance of HIF-1α, PTEN, CD44v6, and survivin for gastric cancer: a meta-analysis. PLoS One. 2014;9:e91842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Zhu CL, Huang Q, Liu CH, Lin XS, Xie F. Prognostic value of HIF-1α expression in patients with gastric cancer. Mol Biol Rep. 2013;40:6055-6062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Zhang ZG, Zhang QN, Wang XH, Tian JH. Hypoxia-inducible factor 1 alpha (HIF-1α) as a prognostic indicator in patients with gastric tumors: a meta-analysis. Asian Pac J Cancer Prev. 2013;14:4195-4198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Chen L, Shi Y, Yuan J, Han Y, Qin R, Wu Q, Jia B, Wei B, Wei L, Dai G. HIF-1 alpha overexpression correlates with poor overall survival and disease-free survival in gastric cancer patients post-gastrectomy. PLoS One. 2014;9:e90678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288:10819-10829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 32. | Xiang L, Gilkes DM, Hu H, Luo W, Bullen JW, Liang H, Semenza GL. HIF-1α and TAZ serve as reciprocal co-activators in human breast cancer cells. Oncotarget. 2015;6:11768-11778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Tawk B, Schwager C, Deffaa O, Dyckhoff G, Warta R, Linge A, Krause M, Weichert W, Baumann M, Herold-Mende C. Comparative analysis of transcriptomics based hypoxia signatures in head- and neck squamous cell carcinoma. Radiother Oncol. 2016;118:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Schmidt S, Linge A, Zwanenburg A, Leger S, Lohaus F, Krenn C, Appold S, Gudziol V, Nowak A, von Neubeck C. Development and Validation of a Gene Signature for Patients with Head and Neck Carcinomas Treated by Postoperative Radio (chemo) therapy. Clin Cancer Res. 2018;24:1364-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Piltti J, Bygdell J, Qu C, Lammi MJ. Effects of long-term low oxygen tension in human chondrosarcoma cells. J Cell Biochem. 2018;119:2320-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Takahashi A, Nakayama R, Ishibashi N, Doi A, Ichinohe R, Ikuyo Y, Takahashi T, Marui S, Yasuhara K, Nakamura T. Analysis of gene expression profiles of soft tissue sarcoma using a combination of knowledge-based filtering with integration of multiple statistics. PLoS One. 2014;9:e106801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Kullmann M, Kalayda GV, Hellwig M, Kotz S, Hilger RA, Metzger S, Jaehde U. Assessing the contribution of the two protein disulfide isomerases PDIA1 and PDIA3 to cisplatin resistance. J Inorg Biochem. 2015;153:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Lovat PE, Corazzari M, Armstrong JL, Martin S, Pagliarini V, Hill D, Brown AM, Piacentini M, Birch-Machin MA, Redfern CP. Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res. 2008;68:5363-5369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Cheng HP, Liu Q, Li Y, Li XD, Zhu CY. The Inhibitory Effect of PDIA6 Downregulation on Bladder Cancer Cell Proliferation and Invasion. Oncol Res. 2017;25:587-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Wu J, Chen XH, Wang XQ, Yu Y, Ren JM, Xiao Y, Zhou T, Li P, Xu CD. ERp19 contributes to tumorigenicity in human gastric cancer by promoting cell growth, migration and invasion. Oncotarget. 2015;6:11794-11805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |