Copyright
©The Author(s) 2018.
World J Gastroenterol. May 14, 2018; 24(18): 1962-1977
Published online May 14, 2018. doi: 10.3748/wjg.v24.i18.1962
Published online May 14, 2018. doi: 10.3748/wjg.v24.i18.1962
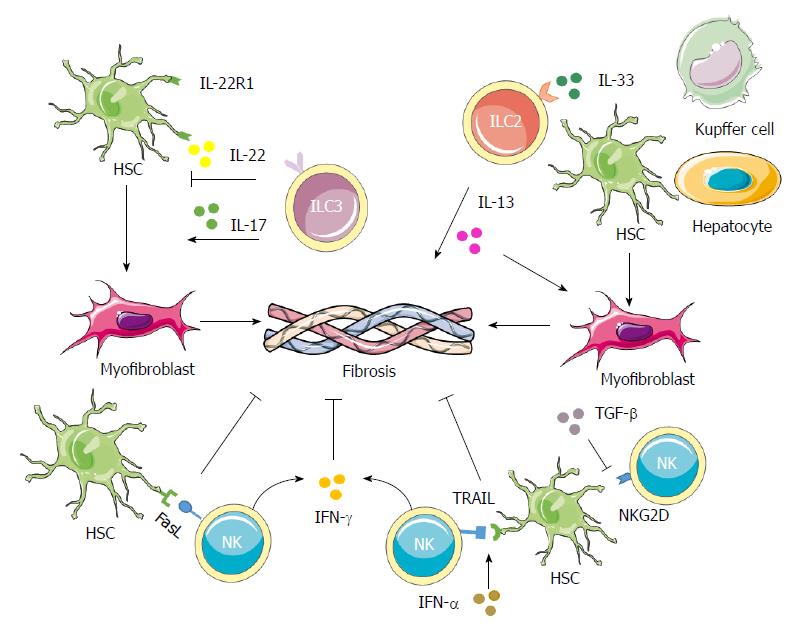
Figure 3 Contributions of innate lymphoid cells in liver fibrosis.
NK cells can decrease the proliferation and activation as well as induce cell cycle arrest of HSCs through IFN-γ. They can also induce the apoptosis of activated HSCs through the TRAIL and Fas ligand pathways. The expression of RAE-1, which is the ligand for the NKG2D activating receptor, is increased on activated HSCs, thus promoting killing by NK cells. IFN-α could increase the surface expression of TRAIL on NK cells to enhance HSCs killing by NK cells, while TGF-β down-regulates the surface expression of NKG2D and 2B4 to suppress the antifibrotic role of NK cells. In both human and mouse cases, IL-33 released from hepatocytes, HSCs and Kupffer cells in response to chronic hepatocellular stress leads to the accumulation and activation of IL-13-producing liver resident ILC2s via ST2-dependent signaling. IL-13 further triggers the activation and transdifferentiation of HSCs into myofibroblasts, to induce potent fibrogenic responses. ILC3s play more complicated roles in fibrosis. On one hand, ILC3s exert an antifibrotic effect by inducing fibroblasts senescence through IL-22 signaling and by down-regulating the expressions of collagen and CTGF through IL-17 signaling. On the other hand, IL-17 can promote inflammation and induce activation of fibroblasts, indicating a profibrotic role for the ILC3s. CTGF: Connective tissue growth factor; HSC: Hepatic stellate cell; IL: Interleukin; IFN: Interferon; NK: Natural killer; TGF: Tumor growth factor; TRAIL: TNF-related apoptosis-inducing ligand.
- Citation: Shen Y, Li J, Wang SQ, Jiang W. Ambiguous roles of innate lymphoid cells in chronic development of liver diseases. World J Gastroenterol 2018; 24(18): 1962-1977
- URL: https://www.wjgnet.com/1007-9327/full/v24/i18/1962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i18.1962