Copyright
©The Author(s) 2018.
World J Gastroenterol. Apr 7, 2018; 24(13): 1478-1485
Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1478
Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1478
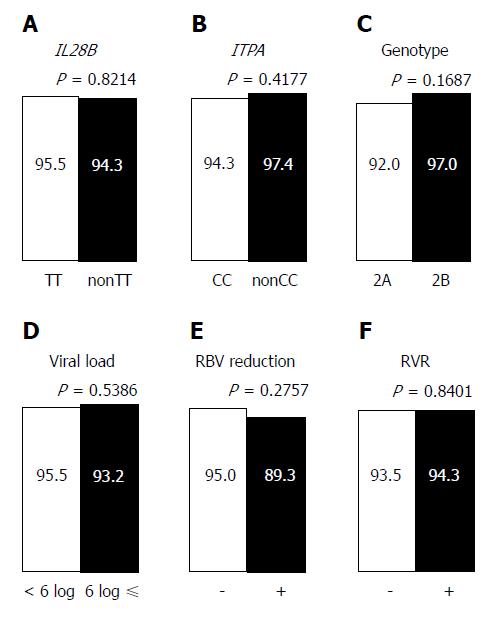
Figure 5 Virological response in patients with sofosbuvir and ribavirin combination therapy categorized by single nucleotide polymorphisms related to anti-hepatitis C virus therapy, pretreatment viral status, ribavirin dose, and rapid virological response.
A: IL28B single nucleotide polymorphisms rs8099917 (TT or non TT); B: ITPA single nucleotide polymorphisms rs1127354 (CC or non CC); C: Hepatitis C virus genotype (2A or 2B); D: Pretreatment viral load (< 6 logIU/mL or ≥ 6 logIU/mL); E: Ribavirin dose reduction (− or +); F: Rapid virological response (− or +). aP < 0.05: Significant difference. IL28B: Interleukin-28B; ITPA: Inosine triphosphate pyrophosphatase; RBV: Ribavirin; RVR: Rapid virological response.
- Citation: Yada M, Miyazaki M, Tanaka K, Masumoto A, Motomura K. Hepatocellular carcinoma or interferon-based therapy history attenuates sofosbuvir/ribavirin for Japanese genotype 2 hepatitis C virus. World J Gastroenterol 2018; 24(13): 1478-1485
- URL: https://www.wjgnet.com/1007-9327/full/v24/i13/1478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i13.1478