Copyright
©The Author(s) 2018.
World J Gastroenterol. Mar 28, 2018; 24(12): 1361-1372
Published online Mar 28, 2018. doi: 10.3748/wjg.v24.i12.1361
Published online Mar 28, 2018. doi: 10.3748/wjg.v24.i12.1361
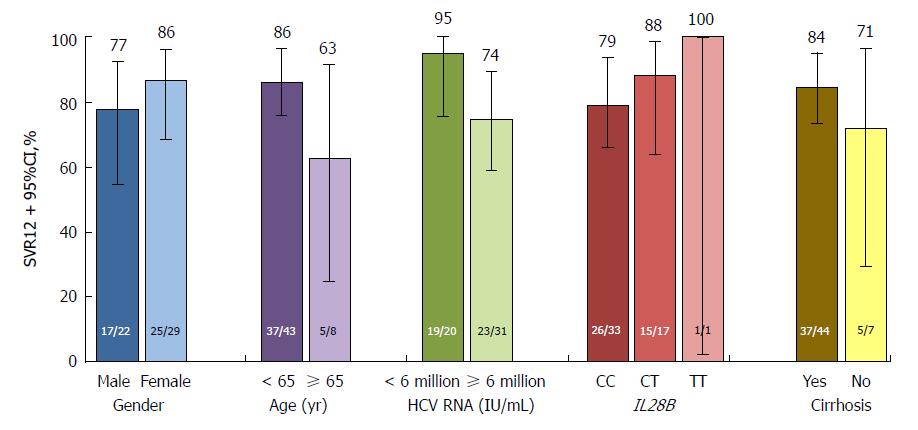
Figure 4 SVR12 according to selected baseline characteristics in the placebo-deferred treatment arm1.
1Reasons for patients not achieving SVR12 included virologic breakthrough (n = 7), relapse (n = 1) or other (n = 1; death, not considered related to study therapy). HCV: Hepatitis C virus; SVR12: Sustained virologic response at post-treatment week 12.
- Citation: Wei L, Wang FS, Zhang MX, Jia JD, Yakovlev AA, Xie W, Burnevich E, Niu JQ, Jung YJ, Jiang XJ, Xu M, Chen XY, Xie Q, Li J, Hou JL, Tang H, Dou XG, Gandhi Y, Hu WH, McPhee F, Noviello S, Treitel M, Mo L, Deng J. Daclatasvir plus asunaprevir in treatment-naïve patients with hepatitis C virus genotype 1b infection. World J Gastroenterol 2018; 24(12): 1361-1372
- URL: https://www.wjgnet.com/1007-9327/full/v24/i12/1361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i12.1361