Copyright
©The Author(s) 2018.
World J Gastroenterol. Mar 28, 2018; 24(12): 1361-1372
Published online Mar 28, 2018. doi: 10.3748/wjg.v24.i12.1361
Published online Mar 28, 2018. doi: 10.3748/wjg.v24.i12.1361
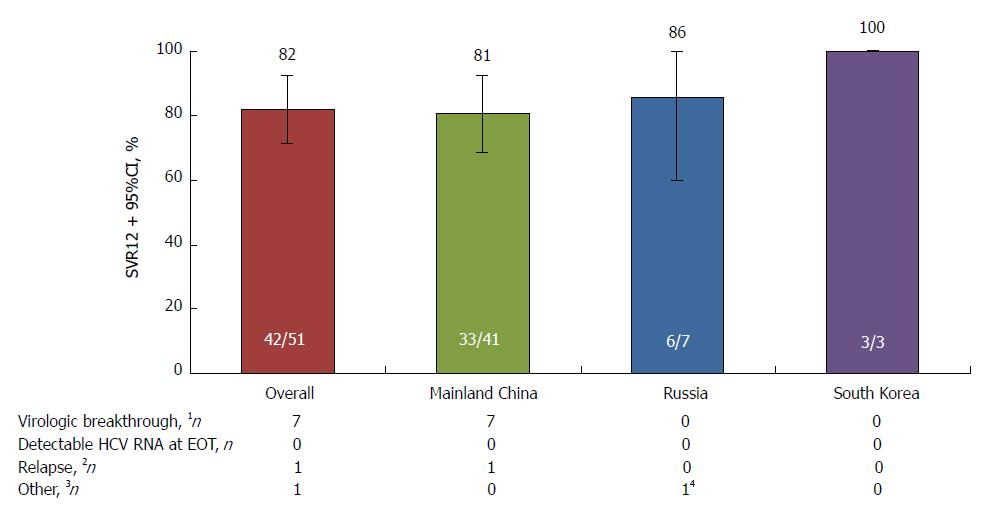
Figure 3 SVR12 in the placebo-deferred treatment arm.
1On-treatment HCV RNA ≥ LLOQ after < LLOQ, or increased >1 log10 over nadir; 2HCV RNA < LLOQ (TND) at EOT followed by HCV RNA ≥ LLOQ at any follow-up visit; 3Other nonresponders included patients who had HCV RNA < LLOQ (TND) at EOT, but with missing posttreatment week 12 data; 4Death, not considered related to study therapy (stab wound). EOT: End of treatment; HCV: Hepatitis C virus; LLOQ: Lower limit of quantitation; SVR12, Sustained virologic response at post-treatment week 12.
- Citation: Wei L, Wang FS, Zhang MX, Jia JD, Yakovlev AA, Xie W, Burnevich E, Niu JQ, Jung YJ, Jiang XJ, Xu M, Chen XY, Xie Q, Li J, Hou JL, Tang H, Dou XG, Gandhi Y, Hu WH, McPhee F, Noviello S, Treitel M, Mo L, Deng J. Daclatasvir plus asunaprevir in treatment-naïve patients with hepatitis C virus genotype 1b infection. World J Gastroenterol 2018; 24(12): 1361-1372
- URL: https://www.wjgnet.com/1007-9327/full/v24/i12/1361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i12.1361