Copyright
©The Author(s) 2017.
World J Gastroenterol. Feb 14, 2017; 23(6): 1090-1097
Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.1090
Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.1090
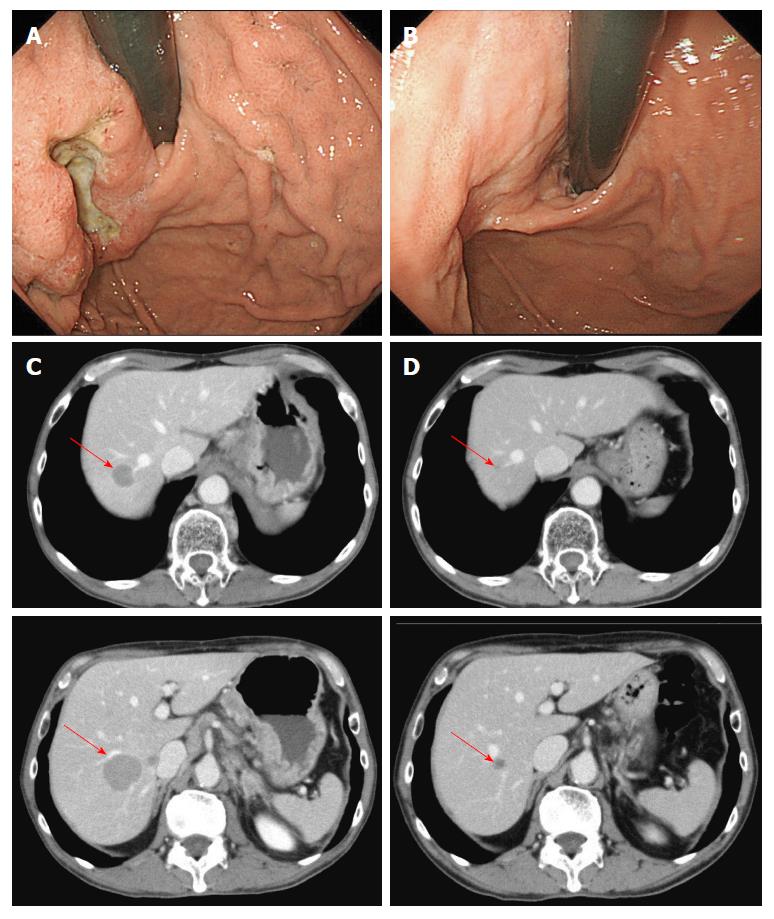
Figure 1 Case 1.
Endoscopic findings (A, B) and computed tomography images (C, D) before (A, C) and after (B, D) seven courses of modified docetaxel, cisplatin and capecitabine (DCX) (mDCX).
- Citation: Maeda O, Matsuoka A, Miyahara R, Funasaka K, Hirooka Y, Fukaya M, Nagino M, Kodera Y, Goto H, Ando Y. Modified docetaxel, cisplatin and capecitabine for stage IV gastric cancer in Japanese patients: A feasibility study. World J Gastroenterol 2017; 23(6): 1090-1097
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/1090.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.1090