Copyright
©The Author(s) 2017.
World J Gastroenterol. Feb 7, 2017; 23(5): 817-829
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.817
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.817
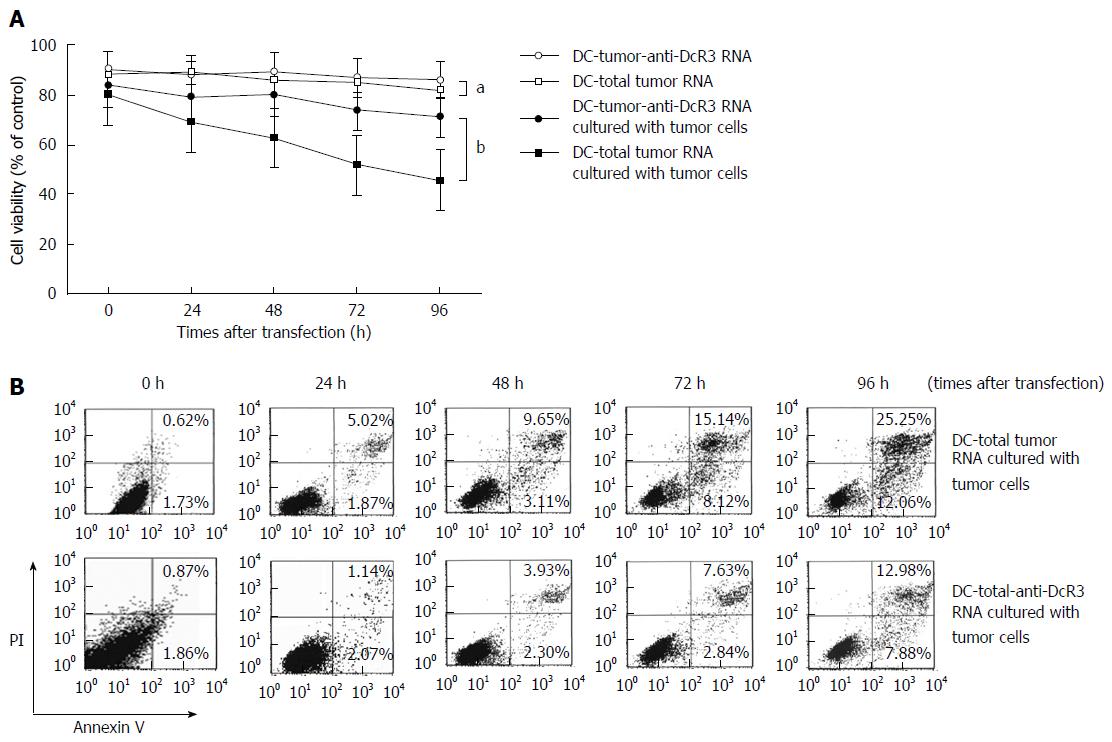
Figure 4 Effects of anti-DcR3 monoclonal antibody secreting dendritic cells on cell viability and apoptosis in tumor RNA-loaded dendritic cells.
A: Dendritic cells (DCs) secreting anti-DcR3 mAb improved the viability of RNA-loaded DCs. The viabilities of DC-total tumor RNA and DC-tumor-anti-DcR3 RNA, cultured with or without autologous tumor cells, were measured using MTT assay after transfection for 0-96 h. The viability of DCs transfected with total tumor RNA alone or together with anti-DcR3 mAb mRNA did not change significantly at the designed time points, with approximately 85% survival throughout (aP > 0.05). Within the same period, the viability of DC-total tumor RNA cultured with tumor cells was evidently lower than that of DC-tumor-anti-DcR3 RNA and decreased in a time-dependent manner (bP < 0.01). Three representative experiments were run, and the data are shown as mean ± SD; B: DC-total tumor RNA and DC-tumor-anti-DcR3 RNA were co-cultured with autologous pancreatic cancer (PC) cells (1 × 106) for 0, 24, 48, 72 and 96 h. Cells stained with annexin V-FITC and propidium iodide were analyzed by flow cytometry. The percentage of annexin V-positive apoptotic cells in DCs transfected with total tumor RNA markedly increased in a time-dependent manner, whereas the apoptotic cells increased slowly in DCs co-transfected with total tumor RNA and anti-DcR3 mAb mRNA. mAb: Monoclonal antibody.
- Citation: Chen J, Guo XZ, Li HY, Zhao JJ, Xu WD. Dendritic cells engineered to secrete anti-DcR3 antibody augment cytotoxic T lymphocyte response against pancreatic cancer in vitro. World J Gastroenterol 2017; 23(5): 817-829
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.817