Copyright
©The Author(s) 2017.
World J Gastroenterol. Feb 7, 2017; 23(5): 817-829
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.817
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.817
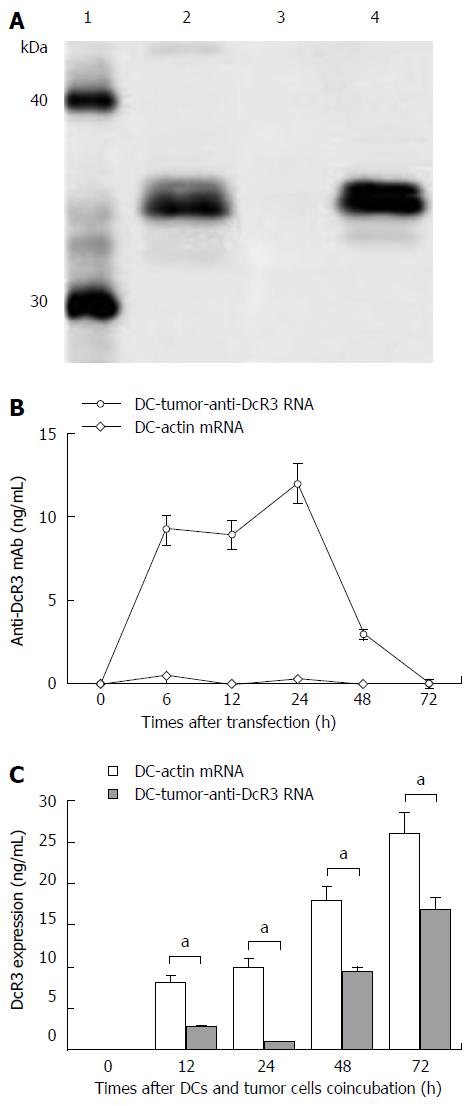
Figure 2 Identification, concentration and function of anti-DcR3 monoclonal antibody secreted by dendritic cells co-transfected with total tumor RNA and anti-DcR3 monoclonal antibody mRNA.
A: Western blotting analysis showed that, similar to commercial anti-DcR3 mAb (lane 2), mAb secreted by dendritic cells (DCs) co-transfected with total tumor RNA and humanized anti-DcR3 H+L mRNA (lane 4) could also react with the recombinant human DcR3 protein (molecular weight of 35 kDa) and generate a band with molecular weight slightly greater than 30 kDa, whereas the supernatant harvested from DC-actin RNA could not bind the DcR3 protein (lane 3); B: The amounts of anti-DcR3 mAb produced by RNA transfected DCs were analyzed using indirect ELISA assay. The mAb secreted by DC-tumor-anti-DcR3 RNA was transient, peaked at 24 h, and then could not be detected after 72 h. However, no anti-DcR3 mAb was found in the supernatant of DC-actin RNA continuously; C: The specific antigen binding effect of anti-DcR3 mAb secreted by RNA transfected DCs was determined by measuring the levels of DcR3 protein in the supernatant of autologous tumor cells (1 × 106 cells) co-cultured with defined DCs (1 × 106 cells). After co-incubation with DC-tumor-anti-DcR3 RNA for 12-72 h, the soluble DcR3 protein level in the supernatant of autologous PC cells was significantly lower than those of tumor cells and the DC-actin RNA co-cultured group (aP < 0.05). Except for those of western blotting and histogram, data represent the means of three experiments, and the histograms are representative of three experiments. Error bars represent SD. mAb: Monoclonal antibody.
- Citation: Chen J, Guo XZ, Li HY, Zhao JJ, Xu WD. Dendritic cells engineered to secrete anti-DcR3 antibody augment cytotoxic T lymphocyte response against pancreatic cancer in vitro. World J Gastroenterol 2017; 23(5): 817-829
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.817