Copyright
©The Author(s) 2017.
World J Gastroenterol. Feb 7, 2017; 23(5): 763-775
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.763
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.763
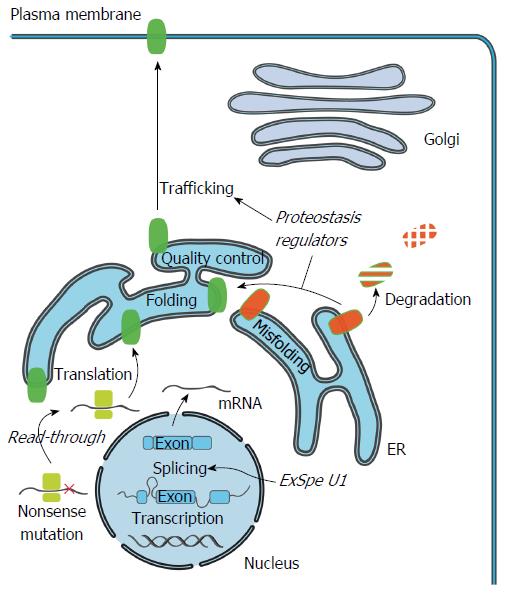
Figure 2 Cellular mechanism of cholestasis-causing mutations and therapeutics.
Specific mutations can impair production of full-length ATP8B1/BSEP/MDR3 protein by induction of premature termination codons (PTC). Compounds such as aminoglycosides and Ataluren, could potentially induce read-through of the PTC and allow translation of full-length transporter protein. Mutations that impair pre-mRNA splicing, protein folding or trafficking can lead to a reduced number or total absence of functional transporters at the cell surface. Pre-mRNA splicing could be restored to enhance the levels of correctly spliced transcripts using modified U1snRNA. Exon specific U1 snRNA (ExSpe U1) provide a special class of modified U1 snRNA molecules that could potentially rescue splicing of multiple mutations in a single exon with less potential side-effects. Misfolded transporters are recognized by the endoplasmic reticulum (ER) quality control system and are targeted for degradation via the ubiquitin-proteasome system. Compounds that modulate the cellular protein homeostasis machinery (proteostasis regulators) can partially rescue the misprocessing, and restore trafficking to the cell surface, largely via unknown mechanisms.
- Citation: van der Woerd WL, Houwen RH, van de Graaf SF. Current and future therapies for inherited cholestatic liver diseases. World J Gastroenterol 2017; 23(5): 763-775
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/763.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.763