Published online Oct 7, 2017. doi: 10.3748/wjg.v23.i37.6833
Peer-review started: January 20, 2017
First decision: March 16, 2017
Revised: August 24, 2017
Accepted: September 6, 2017
Article in press: September 5, 2017
Published online: October 7, 2017
Processing time: 252 Days and 20.4 Hours
To elucidate the role of STAT3 in hepatocarcinogenesis and biliary ductular proliferation following chronic liver injury.
We investigated thioacetamide (TAA)-induced liver injury, compensatory hepatocyte proliferation, and hepatocellular carcinoma (HCC) development in hepatic STAT3-deficient mice. In addition, we evaluated TAA-induced biliary ductular proliferation and analyzed the activation of sex determining region Y-box9 (SOX9) and Yes-associated protein (YAP), which regulate the transdifferentiation of hepatocytes to cholangiocytes.
Both compensatory hepatocyte proliferation and HCC formation were significantly decreased in hepatic STAT3-deficient mice as compared with control mice. STAT3 deficiency resulted in augmentation of hepatic necrosis and fibrosis. On the other hand, biliary ductular proliferation increased in hepatic STAT3-deficient livers as compared with control livers. SOX9 and YAP were upregulated in hepatic STAT3-deficient hepatocytes.
STAT3 may regulate hepatocyte proliferation as well as transdifferentiation into cholangiocytes and serve as a therapeutic target for HCC inhibition and biliary regeneration.
Core tip: Cell transdifferentiation has been identified as one of the crucial sources of organ regeneration. In liver regeneration, both mature hepatocytes and cholangiocytes have been shown to transdifferentiate to each other to maintain hepatic homeostasis. Although STAT3 has been also shown to be implicated in cellular differentiation, the differentiative role of STAT3 in liver regeneration remains unknown. In this study, we found that hepatic-STAT3 deficiency promoted biliary ductular proliferation with upregulation of Yes-associated protein (YAP)/sex determining region Y-box9 (SOX9), crucial regulators of transdifferentiation, in liver injury of mice. This work suggests that STAT3 determines liver cell fate regulating YAP/SOX9 in liver regeneration.
- Citation: Abe M, Yoshida T, Akiba J, Ikezono Y, Wada F, Masuda A, Sakaue T, Tanaka T, Iwamoto H, Nakamura T, Sata M, Koga H, Yoshimura A, Torimura T. STAT3 deficiency prevents hepatocarcinogenesis and promotes biliary proliferation in thioacetamide-induced liver injury. World J Gastroenterol 2017; 23(37): 6833-6844
- URL: https://www.wjgnet.com/1007-9327/full/v23/i37/6833.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i37.6833
Interleukin (IL)-6 family cytokines are indispensable to liver regeneration. The Janus kinase/signal transducers and activators of transcription (JAK/STAT3) pathway is thought to play the central role in signal transduction mediated by IL-6 family. Several target genes of STAT3 have been identified; these genes include cyclin D, c-myc, bcl-xL, and mcl-1, which are essential for cell proliferation and survival. In murine models of liver injury, ablation of STAT3 in liver results in enhanced liver injury and reduced liver regeneration[1]. These findings suggest the protective and proliferative roles of STAT3 in liver regeneration. In addition, STAT3 has been implicated in cellular differentiation, and its activation is associated with differentiation of T cells and macrophages[2]. Studies have demonstrated that STAT3 is essential for the maintenance of pluripotency of embryonic stem (ES) cells[3,4] and self-renewal of tumor-initiating cells in the liver[5]. However, the differentiative role of STAT3 in liver regeneration is questionable.
Both cholangiocytes and hepatocytes are derived from hepatoblasts generated in the foregut endoderm during liver development. The bipotential hepatobiliary stem/progenitor cells, such as small hepatocytes and oval cells, have been shown to exist in adult liver and are thought to contribute to liver regeneration[6]. On the other hand, previous reports have revealed direct transdifferentiation between hepatocytes and cholangiocytes in chronic liver damage[7,8]. Cholangiocytes transdifferentiate into hepatocytes and replenish massive hepatocyte loss[9]. In reverse, cholangiocytes were shown to be generated from transdifferentiating hepatocytes during biliary injury[10]. These evidences suggest that both mature cholangiocytes and hepatocytes play complimentary roles to promote liver regeneration through transdifferentiation. Sex determining region Y-box9 (SOX9), required for the normal differentiation of the biliary tract, is primarily expressed in mature cholangiocytes. However, weak expression of SOX9 was also observed in a small population of hepatocytes, which may display the potential to transdifferentiate into cholangiocytes[11]. Otherwise, cellular fate in the liver is regulated by Yes-associated protein (YAP), an important effector of Hippo pathway[12,13]. YAP depletion in the liver is characterized by cholangiocytes proliferation after biliary obstruction[14]. In contrast, YAP activation in hepatocytes results in their transdifferentiation into cholangiocytes, leading to overgrowth of atypical ductal cells - a process termed as atypical ductular reaction[12]. By interacting with TEAD transcription factor, YAP induces the expression of target genes, including Notch2[15]. NOTCH2 signaling, in turn, induces the expression of its target genes SOX9[12]. Therefore, YAP/SOX9 axis promotes transdifferentiation of hepatocytes into cholangiocytes. However, the mechanism underlying the regulation of YAP/SOX9 axis activation in hepatocytes needs to be elucidated.
To investigate if STAT3 is implicated in hepatocyte proliferation and differentiation in liver injury, we evaluated thioacetamide (TAA)-induced hepatocarcinogenesis and hepatocyte transdifferentiation in mouse with STAT3-deficient liver. STAT3-deficient liver showed profound ductular proliferation with upregulated YAP/SOX9 expression in hepatocytes. Our study indicates that STAT3 regulates transdifferentiation of hepatocytes into biliary cells.
Mice were hosted in the cage whose dimension is 218 mm × 320 mm × 133 mm, in which recycled paper bedding and enrichment that stimulates natural instincts were put, and had free access to water and a standard rodent diet during the experimental period. All mice were maintained under specific pathogen-free conditions, and were monitored every other day. We observed any signs of abnormalities (i.e. marked weight loss or behavior change), but no mice became ill or died prior to the experimental end point. All mice experiments were conducted in strict accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Kurume Institutional Animal Care and Use Committee. Albumin-Cre (Alb-Cre) mice on a C57/BL6 background expressing Cre recombinase under the control of the mouse albumin gene regulatory region and STAT3flox/flox mice on a C57/BL6 background were prepared previously described[16]. To generate hepatic-STAT3 deficient (STAT3Δhep) mice, Alb-Cre mice were crossed with STAT3 flox/flox mice. 4-6 wk male mice weighing 15-20 g were used in this study. For modelling medium- and long-term Thioacetamide (TAA) responses such as inflammation, cirrhosis, and hepatocellular carcinoma (HCC), individual mice received drinking water with TAA (300 mg/L; Wako) for 16 wk (n = 3 for STAT3Δhep mice, n = 4 for control mice (wild-type mice or STAT3 flox/flox mice) and 30 wk (n = 6 for both STAT3Δhep and control mice) respectively. Mice were sacrificed by an overdose of diethyl ether.
Liver specimens were fixed in 10% buffered formalin and embedded in paraffin. Then, consecutive 4-μm sections were cut and stained with hematoxylin and eosin. Histopathological diagnosis was made according to the General Rules for the Clinical and Pathological Study of Primary Liver Cancer edited by the Liver Cancer Study Group of Japan. For evaluation of fibrosis, Azan staining was performed on paraffin-embedded tissue sections using standard methods. The area of fibrosis was quantified using WinRoof image software (version 6.1, Mitani Corporation, Fukui, Japan) and the results were presented as the ratio of the fibrotic area to the total area of the image. We performed immunohistochemical staining of the paraffin-embedded tissue slices using antibodies against; PCNA (PA5-27214; Thermo Fisher Scientific; dilution 1:100), Keratin19 (KRT19; a gift of Atsushi Miyajima; dilution 1:500), SOX9 (ab71762; Abcam, Cambridge, United Kingdom; dilution 1:500) and YAP (ab39361; Abcam, Cambridge, United Kingdom; dilution 1:100). The slices were soaked with 10mM citrate buffer (pH 6.0) and autoclaved at 121°C for 5 min for antigen retrieval. Nonspecific protein staining was blocked by preincubation with Protein Block Serum-Free (DAKO). The slices were then exposed to each primary antibody or rabbit IgG as a negative control at 4°C overnight. After washing, the sections were incubated with Envision secondary antibody labeled with horseradish peroxidase-polymer complexes (DAKO) and stained with DAB. The cellular nuclei were counterstained with hematoxylin.
Isolated hepatocytes and liver tissues were homogenized with RIPA buffer (25 mmol/L Tris-HCl pH 7.6, 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing 1 mmol/L EDTA (pH 8.0), 0.1 mmol/L NaF, 0.1 mmol/L Na3VO4, 100 mmol/L PMSF, 1 mmol/L DTT, 2 × Protease Inhibitor Cocktail (Nacalai Tesque), and 2 × Halt phosphatase inhibitor cocktail (Pierce). Equal proteins (35 µg) were subjected to SDS-PAGE and subsequently transferred onto equilibrated polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, United States). Immunoblotting analysis was performed using antibodies against; SOX9 (ab71762; Abcam, Cambridge, United Kingdom) and YAP (ab39361; Abcam, Cambridge, United Kingdom), phospho (Y357) -YAP (ab62751; Abcam, Cambridge, United Kingdom), Alpha-1-Fetoprotein (AFP) (DAKO, Glostrup, Denmark), phospho(Y416)-Src family (#2101; Cell Signaling Technologies, Beverly, MA, United States), STAT3 (sc 482; Santa Cruz Biotechnology, CA, United States) and GAPDH (sc25779; Santa Cruz Biotechnology, CA, United States).
Total RNA was isolated using the TRIZOL Reagent l (Invitrogen, Carlsbad, CA, United States) and reverse transcription was performed using Superscript III (Invitrogen). Real time PCR was carried out using SYBR Green (Life Technologies) and TaqMan Assays (Life Technologies) with the following primers. GAPDH control forward: 5’-TGACGTGCCGCCTGGAGAAA-3’, GAPDH control reverse: 5’-AGTGTAGCCCAAGATGCCCTTCAG-3’; SOX-9 forward: 5’-TGCAGCACAAGAAAGACCAC-3’, SOX-9 reverse: 5’-TCTCAATGTTGGAGATGACG-3’; KRT19 forward: 5’-GACTTCCTATAGCTATCGCC-3’, KRT19 reverse: 5’-AGTAGGAGGCGAGACGATCA-3’; AFP forward: 5’-GGCCGACATTTTCATTGGACATT-3’, AFP reverse: 5’-TGGGGGAGGGGCATAGGTTTT-3’; MMP2 forward: 5’-GATAACCTGGATGCCGTCGTG-3’, MMP2 reverse: 5’-CTTCACGCTCTTGAGACTTTGGTTC-3’; MMP9 forward: 5’-GCTGACTACGATAAGGACGGC-3’, MMP9 reverse:5’-AGGAAGACGAAGTGAAGGGGAAGA-3’. TaqMan probes/primers for YAP (Mm01143263_m1), IL-6 (Mm00436767_m1), IL-33 (Mm00505403_m1) and Osteopontin (Mm00436767_m1) were purchased from Life Technologies. Expression of mRNA was calculated using the 2-ΔΔCt method. The relative amount of specific mRNA was normalized to GAPDH.
Statistical significance was assessed using the Mann-Whitney U-test. P < 0.05 was considered statistically significant. The data were presented as mean ± SE.
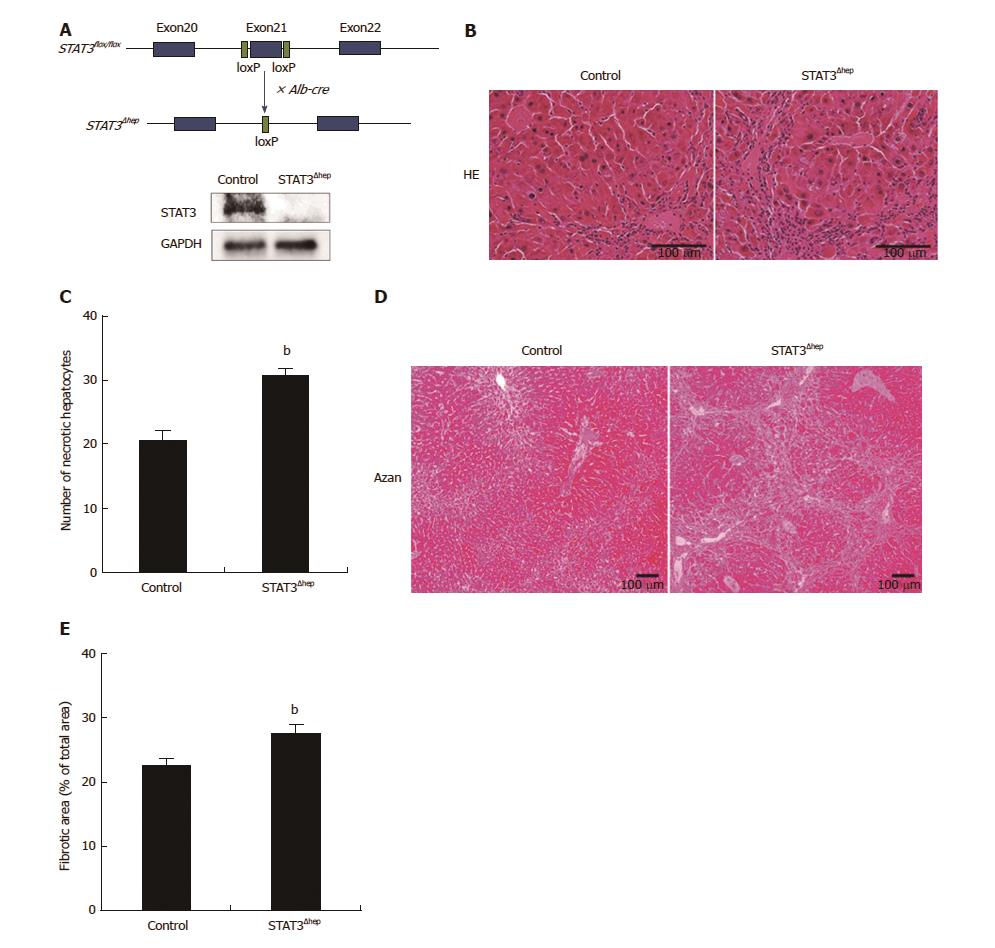
To examine the protective role of STAT3 in hepatocytes, we first examined whether hepatic-STAT3 deficiency promoted TAA-induced liver injury. STAT3flox/flox mice were crossed with Alb-Cre mice to generate hepatic-STAT3 deficient (STAT3Δhep) mice (Figure 1A). STAT3Δhep and control mice were given water with TAA. Metabolites of TAA, which are covalently bound to proteins and lipids, caused hepatocytes necrosis and periportal infiltration of inflammatory cells. As shown Figure 1B, TAA-induced inflammatory infiltrates were augmented in STAT3Δhep mice compared to control mice. Moreover, TAA-induced necrotic changes of hepatocytes were also enhanced in STAT3Δhep mice (Figure 1B and C), indicating that hepatic-STAT3 deficiency promoted TAA-induced hepatocytes injury. As a consequence of enhanced inflammatory change, STAT3Δhep mice exhibited significantly severe liver fibrosis compared to control mice (Figure 1D and E).
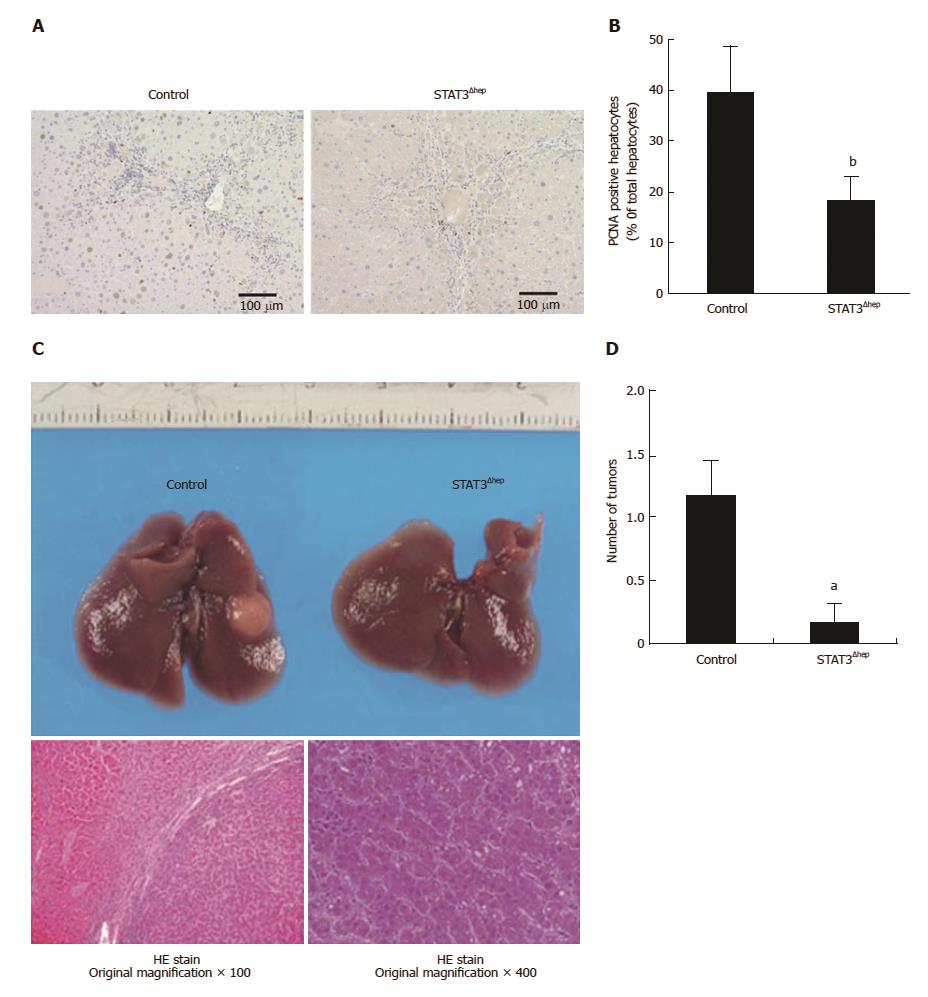
To elucidate the proliferative role of STAT3, we next examined whether TAA-induced hepatocytes regeneration was attenuated in STAT3Δhep mice. Immunohistochemical staining for proliferating cell nuclear antigen (PCNA) showed compensatory proliferation of hepatocytes in TAA-induced liver injury (Figure 2A). PCNA positive hepatocytes were significantly decreased in STAT3Δhep mice (Figure 2A and B), indicative of the proliferative role of hepatic STAT3 in TAA-induced liver injury. Compensatory proliferation triggered by hepatocyte loss leads to not only liver regeneration but also development of HCC. Therefore, we next examined whether TAA-induced HCC development was inhibited in STAT3Δhep mice. TAA treatment for thirty-week developed liver tumors which were histologically consistent with well-differentiated HCC with no evident cholangiocellular carcinoma (Figure 2C). The liver tumor formations of STAT3Δhep mice were significantly lesser than those of control mice (Figure 2D). These findings suggested that STAT3 was required, at least in part, for compensatory hepatocyte proliferation leading to HCC development.
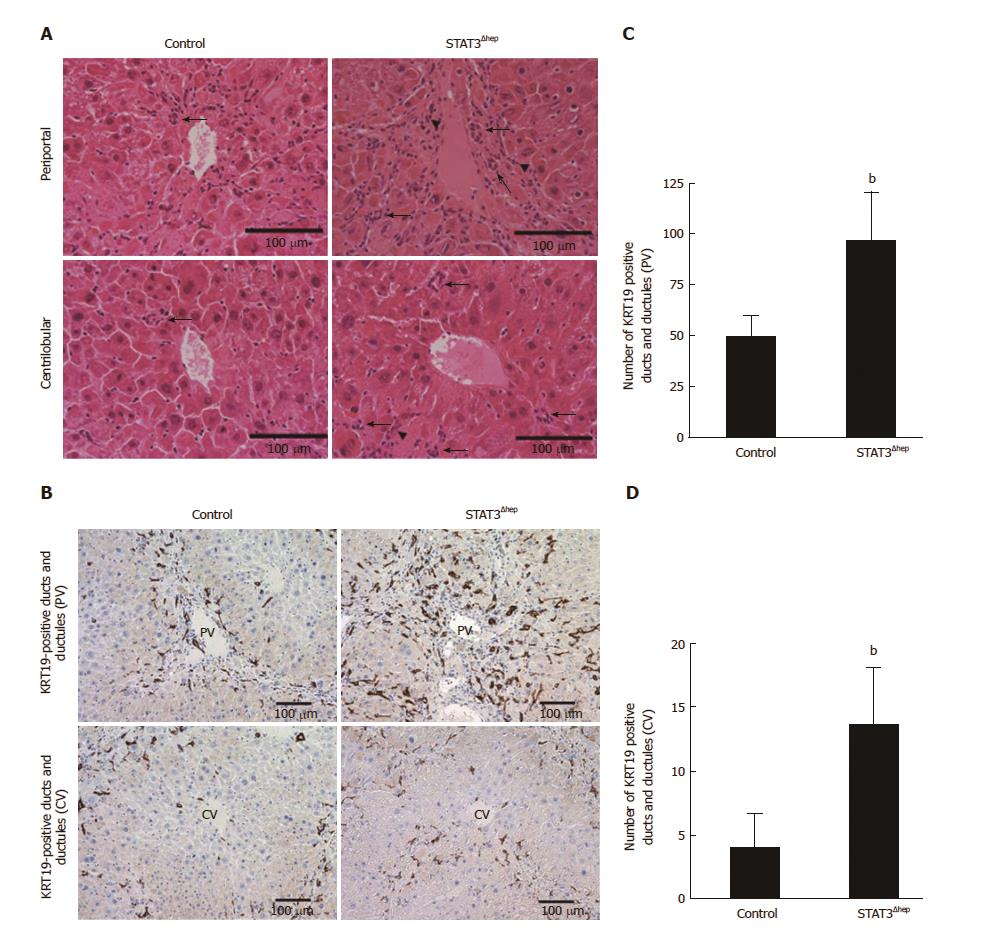
Hepatic necroinflammatory change induces atypical ductular reaction, which is characterized by proliferation of ductular cells. TAA treatment caused the biliary duct/ductular structure formation especially around periportal area (Figure 3A). To confirm the characteristics of bile duct/ductular cells, we performed immunohistochemical staining for KRT19, a marker of mature cholangiocytes. Most of ducts/ductular cells were immunoreactive for KRT19 (Figure 3B). The number of bile duct/ductular structures around periportal area of STAT3Δhep mice was higher than that of control mice (Figure 3C left). The bile duct/ductular formations in centrilobular area were rarely observed in control mice, but those of STAT3Δhep mice were significantly increased (Figure 3C right). KRT19-positive bile ducts/ductular structures were significantly expanded at both periportal and centrilobular area in STAT3Δhep mice (Figure 3C and D).
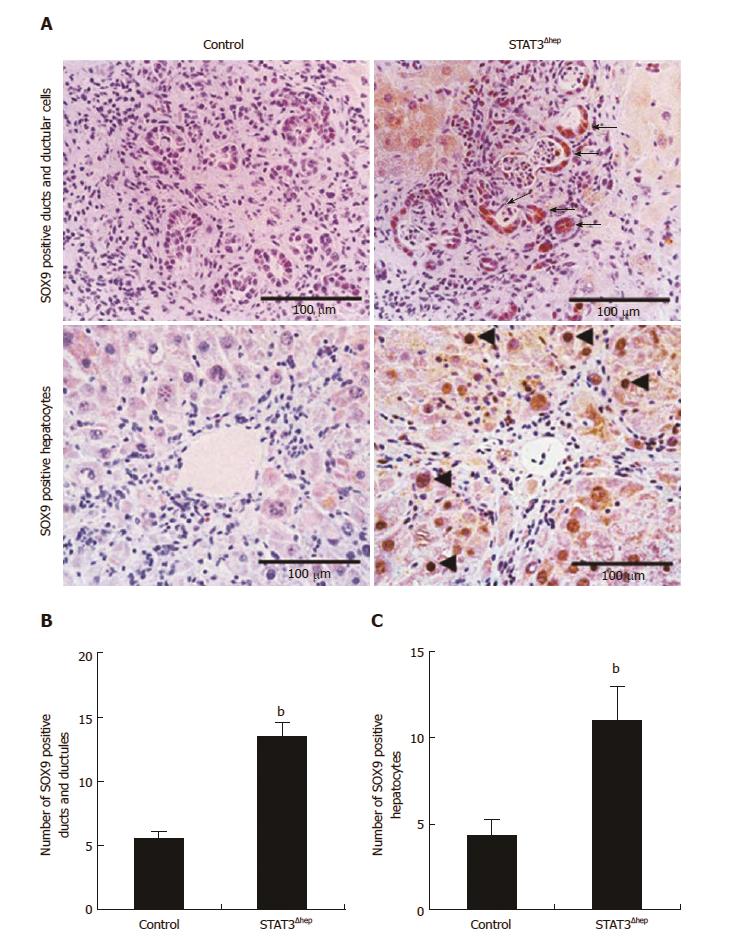
The formation of ducts/ductular structures has been thought to be attributable to both proliferation of mature cholangiocytes and biliary transdifferention of hepatocytes. To examine the origin of bile ducts/ductular structures, we next performed immunohistochemical staining for SOX9, because SOX9 is expressed in cholangiocytes and bipotential hepatobiliary cells[11]. As shown Figure 4A, the bile ducts/ductular structures were frequently composed of SOX9-positive cells. The number of SOX9-positive bile ducts/ductules of STAT3Δhep mice was higher than that of control mice (Figure 4B). Furthermore, immunoreactive intensity for SOX9 was increased in the bile ducts/ductular cells of STAT3Δhep mice compared to control mice. TAA treatment upregulated SOX9 expression in periportal hepatocytes. Interestingly, SOX9 expression was also augmented in STAT3-deficient hepatocytes (Figure 4C).
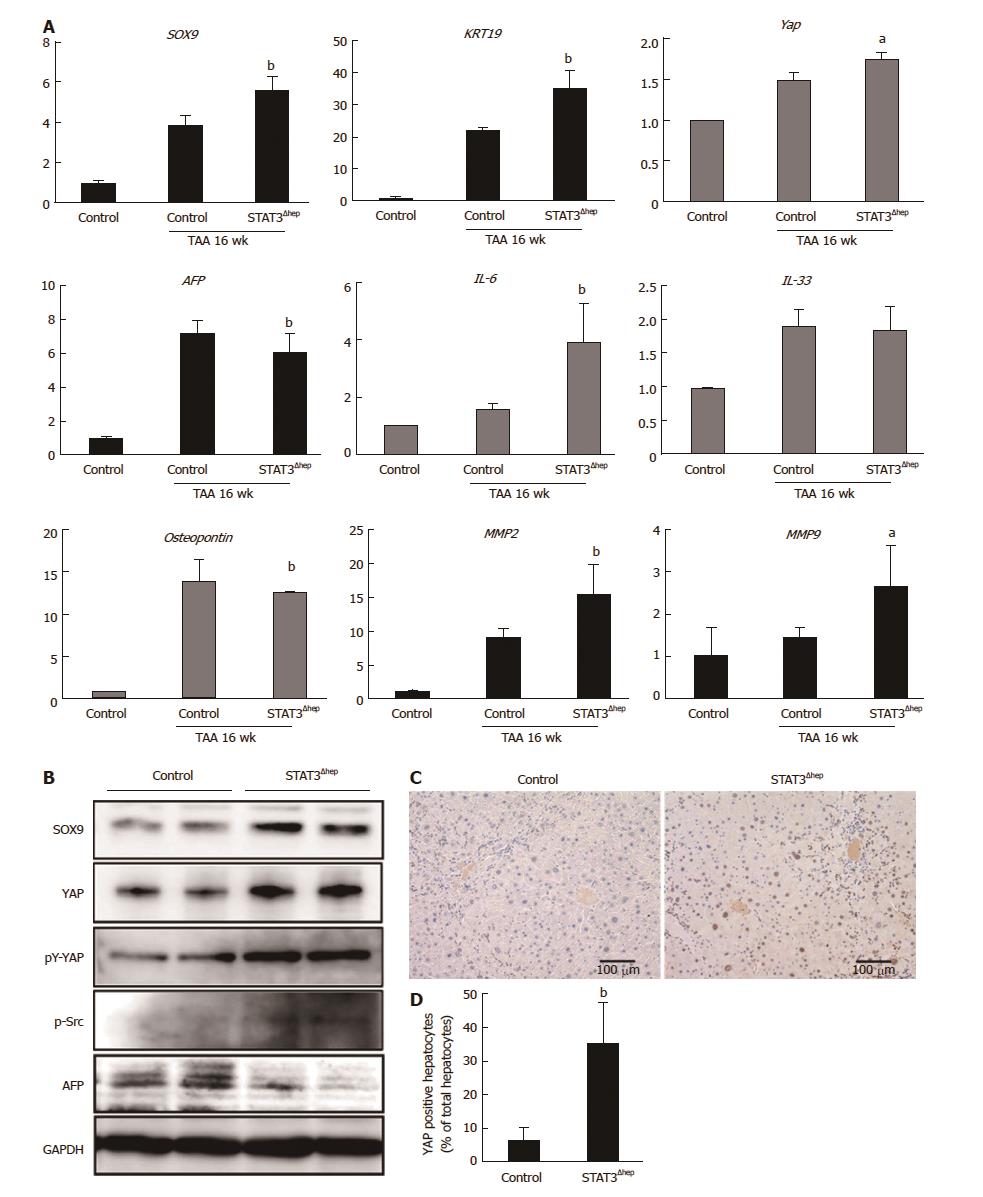
We next performed quantitative RT-PCR analysis to determine whether SOX9 expression was regulated at transcriptional level. TAA treatment upregulated the level of SOX9 mRNA expression, which was significantly increased in the liver of STAT3Δhep mice compared to that of control (TAA 16 wk) (Figure 5A). Immunoblot analysis confirmed higher levels of SOX9 expression in the liver of STAT3Δhep mice (Figure 5B). In accordance with the marked bile duct/ductular cell proliferation, the liver of STAT3Δhep mice showed robust up-regulation of KRT19 expression at transcriptional and protein level. Although IL-33 is a pivotal growth factor for cholangiocytes, IL-33 mRNA was not upregulated in the liver of STAT3Δhep mice (Figure 5A). On the other hand, AFP expression of the liver of STAT3Δhep mice was decreased as compared to that of control mice (Figure 5A and B). Whereas YAP expression was restricted in normal liver, TAA treatment resulted in upregulation of YAP expression. YAP mRNA expression in the liver of STAT3Δhep mice was slightly higher than that of control mice. However, YAP protein expression was markedly increased in the liver of STAT3Δhep mice. Previously, it was shown that src-family kinase induced-YAP tyrosine (Y357) phosphorylation leads to its stabilization and nuclear localization, activating its transcriptional property[17]. YAP tyrosine (Y357) phosphorylation and src-kinase phosphorylation were enhanced in the liver of STAT3Δhep mice. Furthermore, immunohistochemical staining showed robust YAP expression in the nucleus of STAT3-deficient hepatocytes, indicating hepatocytes-transdifferentiation (Figure 5C and D). Taken together, profound proliferation of bile duct/ductular cells in the liver of STAT3Δhep mice may not be due to the proliferation of mature cholangiocytes but may be associated with transdifferentiation of hepatocytes into cholangiocytes.
In this study, we show that STAT3-deficient liver displayed reduced HCC development and promoted biliary ductular proliferation during TAA-induced liver injury. STAT3-deficient hepatocytes exhibited YAP activation and upregulation of SOX9 expression, indicating transdifferentiation of hepatocytes into cholangiocytes. This study is the first report that STAT3 is implicated in both proliferation and differentiation of hepatocytes after liver injury.
IL-6 family cytokines are obviously essential for liver repair. IL-6-deficiency impairs liver regeneration and causes liver failure characterized with blunted DNA synthesis in hepatocytes but not in nonparenchymal cells[18]. The role of JAK/STAT3 pathway has been extensively evaluated and thought to be essential for IL-6-mediated liver repair, because STAT3 regulates many of genes associated with cell survival and proliferation. During liver regeneration after partial hepatectomy, hepatocytes-STAT3 activation was observed in periportal area[19], which might harbor putative stem cell nische[20]. Recently, periportal hepatocytes were shown to have extensive proliferative property for liver repair[11]. These reports suggested that STAT3 activation in periportal area was crucial for liver regeneration. Indeed, TAA-induced liver damage was augmented in liver specific STAT3-deficient mice compared to control mice. However, hepatic STAT3 deficiency did not cause lethal liver injury after TAA treatment. Consistently, previous reports showed that hepatic STAT3 deficiency, unlike IL-6 deficiency, caused modest reduction of liver regeneration with no liver failure after partial hepatectomy[1]. These findings suggest that hepatic STAT3 plays a part of IL-6-mediated liver repair property. Because IL-6 family cytokines activate ERK/MAPK and PI3K/Akt pathways as well as JAK/STAT3 pathway through its receptor gp130, these pathways may lead to compensation of proliferation in STAT3-deficient hepatocytes.
Until recently, YAP has been shown to regulate cell proliferation in some organs including liver[21]. By binding to TEAD transcription factor in nucleus, YAP activates expression of multiple genes including CTGF, CCND1 and BCL2L1 responsible for cell proliferation, antiapoptosis and survival. In Hippo/Lats pathway, YAP serine (S127) phosphorylation results in cytoplasmic sequestration and inhibition of its transcriptional coactivator activity[22,23]. In contrast, src-family kinase induced-YAP tyrosine (Y357) phosphorylation leads to its stabilization and nuclear localization, activating its transcriptional property[17]. Recent report revealed that IL-6-mediated YAP tyrosine (Y357) phosphorylation via a gp130-src family kinase module promotes intestinal epithelial proliferation in vivo[24]. Therefore, YAP and STAT3 cooperatively might promote hepatocytes proliferation and survival for IL-6-mediated liver regeneration. Compensatory YAP activation probably due to IL-6 upregulation might restore the property of STAT3-deficient hepatocytes proliferation and survival. Recently IL-22 has also been shown to be critical for liver regeneration[25]. Although IL-22 does not interact gp130, IL-22 receptor bind SHP2 leading to activation of both src family kinase and STAT3[26]. YAP and STAT3 might be involved in not only IL-6-mediated but IL-22-mediated liver regeneration.
In addition to the role of cell proliferation and survival, YAP sustains undifferentiated state and pluripotency through regulating expression of stemness-associated genes such as Oct4, Sox2 and Nanog[13]. YAP is overexpressed in cultured ES cells and may be required for self-renewal and suppression of differentiation[27]. YAP activation was shown to dedifferentiate mature hepatocytes into hepatobiliary progenitor cells[12]. SOX9-positive periportal hepatocytes display high regenerative property and were shown to self-renew and transdifferentiate into cholangiocytes[11]. It is interesting that SOX9 expression is upregulated by YAP activation through NOTCH pathway[12]. Therefore, activation of YAP/SOX9 axis is critical for hepatocyte dedifferentiation to accomplish liver repair. However, the regulation of YAP/SOX9 axis activation in hepatocytes is still unknown. In this study, we found that STAT3 deficiency enhanced YAP tyrosine (Y357) phosphorylation and SOX9 expression through Src activation during TAA-induced liver injury. These findings suggest that STAT3 may inhibit dedifferentiation and transdifferentiation of hepatocytes by preventing the activation of YAP/SOX9 axis.
Demetris et al[28] previously reported that KRT19 positive/AFP negative hepatocytes, called ductular hepatocytes, appeared to exhibit ductular reaction in submassive liver necrosis. The ductular hepatocytes were thought to contribute to biliary repair through transdifferentiation into cholangiocytes. As AFP synthesis and secretion were derived from differentiated hepatocytes in liver injury, TAA treatment upregulated AFP expression reflecting hepatocytes repopulation. The TAA treatment-induced AFP upregulation was significantly inhibited in hepatic-STAT3 deficient mice, whereas the expression of KRT19 was upregulated compared to control mice. Interestingly, AFP expression is suppressed by YAP in undifferentiated ES cells, and YAP inhibition results in AFP expression with differentiation of ES cell[27]. These findings suggest that YAP activation in STAT3-deficient hepatocytes might direct their transdifferentiation into cholangiocytes instead of their dedifferentiation into progenitor cells. An important question is whether YAP activation in STAT3-deficient hepatocytes contribute to development of liver cancer, as YAP has been found to be involved in cholangiocarcinoma[29]. However, hepatic-STAT3 deficient mice did not cause cholangiocarcinoma, suggesting that STAT3 deficiency-induced YAP activation is insufficient for oncogenic transformation at least in the model of TAA-induced liver injury. Otherwise, hepatic STAT3 depletion promoted fibrotic regeneration in TAA-induced liver injury model. Recent report showed that YAP expression was positively correlated with liver fibrosis in non-alcoholic steatohepatitis[30]. Because YAP has also been found to promote epithelial-mesenchymal transition[31], the possibility is not excluded that YAP activation might promote transdifferentiation of hepatocytes into myofibroblast leading to liver fibrosis.
In conclusion, STAT3 is not only involved in HCC development but also prevents biliary ductular formation in TAA-induced liver injury. Our study highlights hepatic STAT3 as a plausible target for biliary repair during liver injury.
We thank Yasuko Imamura and Masako Hayakawa for excellent technical help, and Taeko Narisawa for secretarial assistance.
JAK/STAT3 pathway plays the central role in signal transduction mediated by IL-6 family, indispensable cytokines for liver regeneration. Several target genes of STAT3 are essential for cell proliferation and survival. In murine models of liver injury, ablation of STAT3 in liver results in enhanced liver injury and reduced liver regeneration.
Cholangiocytes transdifferentiate into hepatocytes and replenish massive hepatocyte loss. In addition, cholangiocytes are generated from transdifferentiating hepatocytes during biliary injury. STAT3 is implicated in cellular differentiation of some immune cells, although the role of STAT3 in transdifferentiation between hepatocytes and cholangiocytes has not been elucidated.
This study indicates that STAT3 regulates transdifferentiation of hepatocytes into biliary cells through Yes-associated protein (YAP)/Sex determining region Y-box9 (SOX9) axis activation.
Hepatic STAT3 is a plausible target for biliary repair during liver injury.
YAP, an important effector of Hippo pathway which controls organ size in animals through the regulation of cell proliferation and apoptosis. A recent report revealed that YAP activation in hepatocytes results in their transdifferentiation into cholangiocytes. SOX9, one of transcription factors which is required for the normal differentiation of the biliary tract. It was recently reported that SOX9-positive periportal hepatocytes transdifferentiate into cholangiocytes and that SOX9 expression is upregulated by YAP activation. Transdifferentiation, a process in which one mature somatic cell converts into another mature somatic cell without going through an intermediate pluripotent or progenitor cell type.
The authors provided evidence showing that STAT3 may mediate carcinogen-induced hepatocellular carcinoma in mouse model. Hepatocyte-specific deletion of STAT3 reduced compensatory proliferation and cancer formation in response to TAA. Additional test indicated that deficiency of STAT3 enhanced YAP activation and SOX9 expression, which may contribute to the enhanced formation of biliary ductular structures. The manuscript was well-written and the results are straightforward.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen F, Zhang L S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Wang H, Park O, Lafdil F, Shen K, Horiguchi N, Yin S, Fu XY, Kunos G, Gao B. Interplay of hepatic and myeloid signal transducer and activator of transcription 3 in facilitating liver regeneration via tempering innate immunity. Hepatology. 2010;51:1354-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Rébé C, Végran F, Berger H, Ghiringhelli F. STAT3 activation: A key factor in tumor immunoescape. JAKSTAT. 2013;2:e23010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Raz R, Lee CK, Cannizzaro LA, d’Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 1999;96:2846-2851. [PubMed] [Cited in This Article: ] |
| 4. | Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048-2060. [PubMed] [Cited in This Article: ] |
| 5. | Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 471] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 6. | Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 264] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 9. | He J, Lu H, Zou Q, Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. 2014;146:789-800.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 10. | Chen YH, Chen HL, Chien CS, Wu SH, Ho YT, Yu CH, Chang MH. Contribution of Mature Hepatocytes to Biliary Regeneration in Rats with Acute and Chronic Biliary Injury. PLoS One. 2015;10:e0134327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, Taniguchi K, Nakagawa H, Valasek MA, Ye L. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162:766-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 12. | Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 632] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 13. | Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054-2060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 900] [Cited by in F6Publishing: 988] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 14. | Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, Cornish T, Alpini G, Bronk S. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097-1107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530-1542.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 16. | Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 656] [Cited by in F6Publishing: 740] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 17. | Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457-1473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 619] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 18. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [PubMed] [Cited in This Article: ] |
| 19. | Sakuda S, Tamura S, Yamada A, Miyagawa Ji, Yamamoto K, Kiso S, Ito N, Imanaka K, Wada A, Naka T, Kishimoto T, Kawata S, Matsuzawa Y. Activation of signal transducer and activator transcription 3 and expression of suppressor of cytokine signal 1 during liver regeneration in rats. J Hepatol. 2002;36:378-384. [PubMed] [Cited in This Article: ] |
| 20. | Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47:1994-2002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 21. | Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 620] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 22. | Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1593] [Cited by in F6Publishing: 1842] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 23. | Shimomura T, Miyamura N, Hata S, Miura R, Hirayama J, Nishina H. The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity. Biochem Biophys Res Commun. 2014;443:917-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 501] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 25. | Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74-G80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Meng S, Gui Q, Xu Q, Lu K, Jiao X, Fan J, Ge B, Ke Y, Zhang S, Wu J. Association of Shp2 with phosphorylated IL-22R1 is required for interleukin-22-induced MAP kinase activation. J Mol Cell Biol. 2010;2:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 590] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 28. | Demetris AJ, Seaberg EC, Wennerberg A, Ionellie J, Michalopoulos G. Ductular reaction after submassive necrosis in humans. Special emphasis on analysis of ductular hepatocytes. Am J Pathol. 1996;149:439-448. [PubMed] [Cited in This Article: ] |
| 29. | Pei T, Li Y, Wang J, Wang H, Liang Y, Shi H, Sun B, Yin D, Sun J, Song R. YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget. 2015;6:17206-17220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Machado MV, Michelotti GA, Pereira TA, Xie G, Premont R, Cortez-Pinto H, Diehl AM. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J Hepatol. 2015;63:962-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405-12410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 673] [Cited by in F6Publishing: 743] [Article Influence: 41.3] [Reference Citation Analysis (0)] |