Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6448
Peer-review started: June 13, 2017
First decision: July 17, 2017
Revised: July 26, 2017
Accepted: August 25, 2017
Article in press: August 25, 2017
Published online: September 21, 2017
Processing time: 100 Days and 20.8 Hours
To compare the value of contrast-enhanced abdominal computed tomography (CT) and fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) for detecting gastric carcinoma recurrence.
We retrospectively examined data from 2475 patients who underwent both contrast-enhanced abdominal CT and FDG PET/CT for the surveillance of gastric carcinoma curative resection. Patients had an interval of less than 1 mo between their CT and PET/CT scans. Sixty patients who had recurrence were enrolled. Among 1896 patients who did not have recurrence, 60 were selected by simple random sampling. All CT and PET/CT images were reviewed retrospectively by two reviewers blinded to all clinical and pathologic information except curative resection due to gastric carcinoma.
The pathological stage of the recurrence group was statistically significantly higher than that of the control group (P < 0.001). In the 60 patients who had recurrence, there were 79 recurrent lesions. Forty-four patients had only one location of recurrence, 13 patients had two locations, and 3 patients had three. In the detection of patient-based overall recurrence, no statistically significant differences existed between the two modalities (P = 0.096). However, for peritoneal carcinomatosis, CT had a statistically significantly higher sensitivity compared to PET/CT (96% vs 50%, P = 0.001). Adenocarcinoma was the most common type of gastric carcinoma. On the pathology-based analysis, CT also had a statistically significantly higher sensitivity compared to PET/CT (98% vs 80%, P = 0.035).
Contrast-enhanced CT was superior to PET/CT in the detection of peritoneal carcinomatosis and pathologic type of adenocarcinoma.
Core tip: Contrast-enhanced abdominal computed tomography (CT) and positron emission tomography/computed tomography (PET/CT) are commonly used imaging methods for surveillance of recurrence after gastric cancer surgery. However, the ideal method for early detection of gastric carcinoma recurrence remains controversial. In this study, we compared the value of contrast-enhanced abdominal CT and PET/CT for detecting the recurrence of gastric carcinoma after curative resection. We found that contrast-enhanced CT was superior to PET/CT in the detection of peritoneal carcinomatosis and pathologic type of adenocarcinoma.
- Citation: Kim JH, Heo SH, Kim JW, Shin SS, Min JJ, Kwon SY, Jeong YY, Kang HK. Evaluation of recurrence in gastric carcinoma: Comparison of contrast-enhanced computed tomography and positron emission tomography/computed tomography. World J Gastroenterol 2017; 23(35): 6448-6456
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6448.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6448
Gastric carcinoma is a leading cause of cancer death worldwide[1]. In Asia, the incidence of gastric carcinoma is high. Its 5-year survival rate is 55%-66%, and a major cause of death after curative surgery for gastric carcinoma is recurrence with a substantial number of patients experiencing such recurrence[2-4]. Although the recurrence rate of early gastric cancer after curative resection is very low, advanced gastric cancer cases show a high recurrence rate after curative resection[5,6].
Several methods exist to detect the recurrence of gastric carcinoma after surgery, including tumor markers, endoscopy, and imaging studies[7]. Elevated tumor marker levels such as those of carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) can be associated with tumor recurrence[8]. Non-systemically, endoscopy can be used to detect locoregional tumor recurrence[9]. Contrast-enhanced abdominal computed tomography (CT) and fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) are cross-sectional imaging modalities that can be used to detect locoregional and distant recurrence. However there is no specific guideline or indication for using CT or PET/CT in surveillance for recurrence after gastric cancer surgery.
Contrast-enhanced abdominal CT is a commonly used imaging method because it is widely available and relatively inexpensive[10]. Despite its widespread use, however, there have been controversies about the role of CT, compared to PET/CT, in evaluating the recurrence of gastric carcinoma after curative surgery. Previous reports showed that PET or PET/CT might be useful in the evaluation of the recurrent gastric cancer after gastric cancer surgery[10-12]. Additional use of PET/CT in contrast-enhanced CT studies could improve the detection of recurrence and provide other information such as an unexpected secondary malignancy[7]. On the other hand, PET or PET/CT might not be suitable for the detection and confirmation of recurrence after gastric cancer surgery[13,14]. Some reports have shown that contrast-enhanced CT and PET/CT have no substantial differences in their ability to detect gastric cancer recurrence after curative resection, except for detecting peritoneal carcinomatosis[7].
Thus, the ideal method for early detection of gastric carcinoma recurrence remains controversial[10,11,13-15]. Generally, previous studies reviewed images without being blinded to the clinical findings and results of the imaging studies, or only evaluated PET or PET/CT for detecting recurrence after gastric surgery[10-12,14,16,17].
The aim of this study was to compare the efficacy of CT and PET/CT in detecting recurrence after curative gastric surgery, when using blinded clinical and imaging findings.
This retrospective study was approved by the Institutional Review Board. Informed consent was waived because of the retrospective nature of the study. The data of 2475 patients who subsequently underwent both contrast-enhanced abdominal CT and PET/CT for the surveillance of gastric carcinoma recurrence after curative resection, from December 2011 to June 2015, were analyzed. Patients all had an interval of less than 1 mo between their CT and PET/CT scans. Of the 2475 patients, 519 who did not have a diagnosis validated by histopathological confirmation or subsequent imaging study least 6 mo were excluded. Sixty patients had recurrence confirmed by histopathologic evaluation or subsequent imaging study least 6 mo. Twenty eight patients had pathologic confirmation and 32 patients had imaging confirmation by serial contrast-enhanced CT, making a total of 60 patients. Radiologically, recurrence was defined to be present when a suspicious lesion showed the interval increment in size during serial imaging studies. In the remaining 1896 patients without recurrence, a control group of 60 patients was selected by simple random sampling (Figure 1). In case of patients who had recurrence and underwent both CT and PET/CT many times with intervals of less than 1 mo, the first CT or PET/CT images suspected to show recurrence were analyzed. In case of patients who had no recurrence and underwent both CT and PET/CT many times with intervals of less than 1 mo, the first CT and PET/CT images were analyzed.
Contrast-enhanced abdominal CT was performed with either 16 or 64-detector row CT scanner (LightSpeed 16 or LightSpeed VCT; GE Healthcare, Milwaukee, WI, United States) with the following parameters; 120 kV, 200-300 mA, slice thickness: 3.75 mm, slice increments: 3.75 mm, and pitch: 0.984:1. Automated tube current modulation (AutomA; GE Healthcaere, Milwaukee, WI, United States) was routinely used for all patients. AutomA was set between 100 and 300 mA with a noise index of 15. Single-phase (portal venous phase) contrast-enhanced CT was performed after intravenous injection of 100-150 mL of iopromide (Ultravist®; Schering, Berlin, Germany) at a rate of 2-3 mL/s. The total amount of injected contrast material was adjusted according to the body weight of the patients (2 mL/kg). Scanning was started 90 s after the intravenous injection, spanning from the liver dome to the symphysis pubis.
18F-FDG PET/CT was performed with a Discovery ST PET/CT system (GE Healthcare), consisting of a bismuthgermanate full scanner and a 16-detector-row CT scanner. The patients fasted for at least 6 h prior to the intravenous administration of 18F-FDG (7.4 MBq per body weight). At 60 min after 18F-FDG administration, transmission data were acquired using low-dose CT (120 kV, automated from 10 to 130 mA, 50 cm field of view (FOV), a scan length of 40-50 s and a slice thickness of 3.75 mm, and a rotation time of 0.7 s), extending from the base of the skull to the proximal thighs. Immediately after CT acquisition, PET emission scans were acquired in the same anatomic locations with a 15.7 cm axial FOV acquired in the two-dimensional mode with 3 min/bed position. The CT data were used for attenuation correction. The images were reconstructed using a conventional iterative algorithm. A workstation (Xeleris) providing multi-planar reformatted images was also used for image display and analysis.
All CT and PET/CT images were reviewed retrospectively, and recurrence was defined by the consensus of two experienced abdominal radiologists (22 and 12 years of experience) and two experienced nuclear medicine physicians (20 and 12 years of experience). Reviewers were blinded to all clinical and pathological information about the patients except for their curative resection of gastric carcinoma.
We classified recurrent lesions into 5 categories: (1) Locoregional recurrence; (2) Lymph node recurrence; (3) Liver metastasis; (4) Peritoneal carcinomatosis; and (5) Other recurrence, for recurrence not included in categories 1-4.
In CT images, locoregional recurrence was defined as showing wall thickening with contrast enhancement of the anastomotic site and adjacent remnant stomach[18]. Lymph nodes more than 10 mm long in the short axis were considered lymph node recurrence. A nearly round shape, spiculated or indistinct borders, central necrosis, and marked or heterogeneous enhancement were integrated with the CT size criteria[19]. Liver metastasis was defined as a hypoattenuating lesion with peripheral rim enhancement[20]. Nodular-plaque or infiltrative soft tissue stranding in the mesentery or omentum, parietal peritoneal thickening and contrast enhancement with or without ascites was considered peritoneal carcinomatosis[21].
Focal hypermetabolic activity (> 3.0 SUV) in the anastomotic site and adjacent remnant stomach was considered locoregional recurrence[7]. Lymph nodes were graded as malignant or benign based on functional criteria (increased metabolism relative to the surrounding lymph nodes) independent of their size[22]. Focal hypermetabolic activity greater than adjacent normal liver was considered liver metastasis[23]. Diffuse hypermetabolism spreading throughout the abdominopelvic cavity, obscuring visceral outlines and randomly located discrete foci of uptake anteriorly within the abdomen or dependently within the pelvis and unrelated to solid viscera or nodal stations was considered peritoneal carcinomatosis[19].
Data from the reviewers were analyzed separately. The characteristics of patients with and without recurrence were compared using Student’s t test for noncategorical variables and the χ2 test for categorical variables. Sensitivity, specificity, and accuracy were calculated. McNemar’s test was conducted for comparing the diagnostic efficacy of contrast-enhanced abdominal CT and PET/CT in patients-, lesion- and pathological type-based analysis. A P-value of < 0.05 was considered to indicate a significant difference.
Table 1 details the patient characteristics. The pa-thological stage of the recurrence group was statistically significantly higher than that of the control group (P < 0.001). In the control group, the stage of 33 patients (55%) was Ia and none of the patients was IV. In the recurrence group, none of the patients was Ia and seven patients were. These patients did not have distant metastasis in their preoperative clinical stage. After the curative surgery, five patients had distant lymph node metastasis and two patients had peritoneal metastasis as revealed by peritoneal washing cytology. The median interval between contrast-enhanced CT and PET/CT was statistically significantly longer in the recurrence group than in the control group (P < 0.001). Other patient characteristics did not differ.
| Characteristics | Recurrence group (n = 60) | Control group (n = 60) | ||
| P value | ||||
| Age (yr) | Median | 60.6 | 65 | 0.755 |
| Range | 29-80 | 35-85 | ||
| Sex | Male | 37 (62) | 16 (27) | 0.172 |
| Female | 23 (38) | 44 (73) | ||
| Operation | Total gastrectomy | 17 (28) | 12 (20) | 0.286 |
| Subtotal gastrectomy | 43 (72) | 48 (80) | ||
| AJCC stage | IA | 0 (0) | 33 (55) | < 0.001 |
| IB | 5 (8) | 10 (17) | ||
| IIA | 7 (12) | 9 (15) | ||
| IIB | 9 (15) | 3 (5) | ||
| IIIA | 7 (12) | 1 (2) | ||
| IIIB | 10 (16) | 2 (3) | ||
| IIIC | 15 (25) | 2 (3) | ||
| IV | 7 (12) | 0 (0) | ||
| Pathology | Adenocarcinoma | 51 (85) | 55 (92) | 0.454 |
| Signet ring cell carcinoma | 5 (8) | 2 (3) | ||
| Mucinous adenocarcinoma | 4 (7) | 3 (5) | ||
| Interval between CT and PET/CT (d) | Median | 5.9 | 2.8 | < 0.001 |
| Range | 0-28 | 0-9 | ||
In the 60 patients who had recurrence, there were 79 recurrent lesions (Table 2). Forty-four patients had only one location of recurrence, 13 patients had two locations, and 3 patients had three. Locations of the other recurrence sites (n = 18) were intestines (n = 7), bone (n = 5), ovaries (n = 3), spleen (n = 2), and pancreas (n = 1).
For the detection of patient-based overall recurrence, CT had a sensitivity of 97%, a specificity of 97%, and an accuracy of 97%, whereas PET/CT had a sensitivity of 82%, a specificity of 95%, and an accuracy of 88%. These differences were not statistically significant (P = 0.096; Table 3). Two patients with recurrence did not have it detected by CT, and 12 patients who had recurrence did not have it detected by PET/CT.
| Site | Imaging | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) | P value |
| Overall | CT | 97 (58/60) | 97 (58/60) | 97 (58/60) | 97 (58/60) | 97 (114/120) | 0.096 |
| PET/CT | 82 (49/60) | 95 (57/60) | 94 (49/52) | 84 (57/68) | 88 (106/120) | ||
| Locoregional | CT | 80 (8/10) | 100 (110/110) | 100 (8/8) | 98 (112/110) | 98 (118/120) | 1.000 |
| PET/CT | 80 (8/10) | 99 (109/110) | 89 (8/9) | 98 (109/111) | 98 (117/120) | ||
| Lymph node | CT | 92 (22/24) | 99 (95/96) | 96 (22/23) | 98 (95/97) | 98 (117/120) | 1.000 |
| PET/CT | 88 (21/24) | 99 (95/96) | 95 (21/22) | 97 (95/98) | 97 (116/120) | ||
| Liver | CT | 67 (2/3) | 96 (112/117) | 29 (2/7) | 99 (112/113) | 95 (114/120) | 0.688 |
| PET/CT | 100 (3/3) | 98 (115/117) | 60 (3/5) | 100 (115/115) | 98 (118/120) | ||
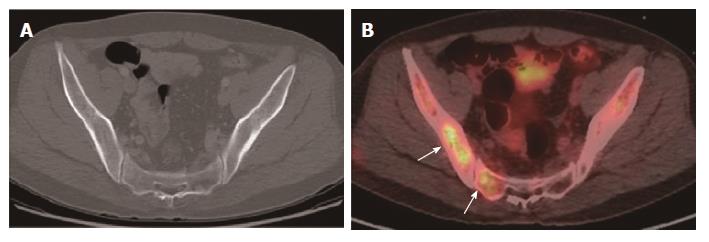
| Peritoneal carcinomatosis | CT | 96 (23/24) | 100 (96/96) | 100 (23/23) | 99 (96/97) | 99 (119/120) | 0.001 |
| PET/CT | 50 (12/24) | 100 (96/96) | 100 (12/12) | 89 (96/108) | 90 (108/120) | ||
| Total lesion | CT | 86 (68/79) | 98 (511/521) | 87 (68/78) | 98 (511/522) | 97 (579/600) | 0.089 |
| PET/CT | 76 (60/79) | 98 (513/521) | 88 (60/68) | 96 (513/532) | 96 (573/600) |
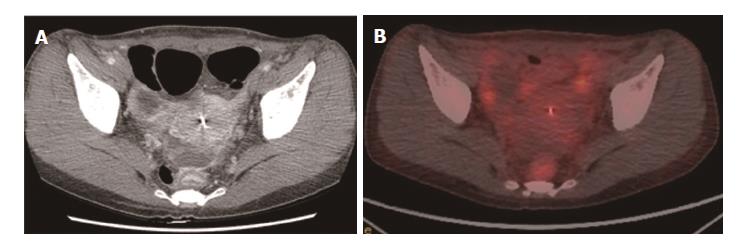
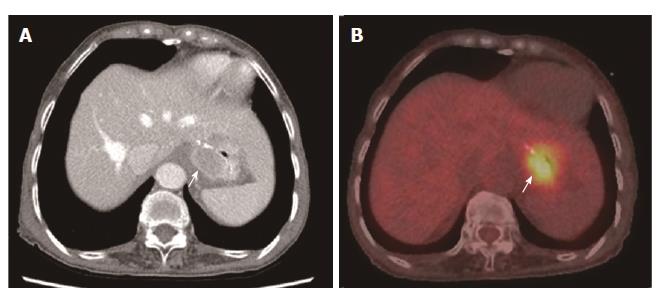
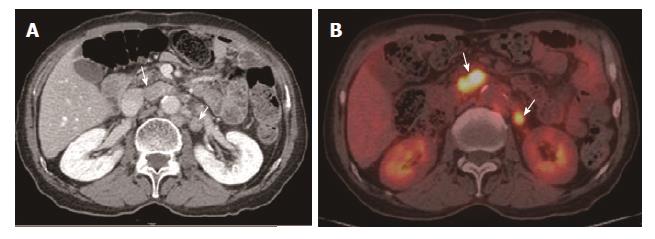
For the detection of lesion-based overall recurrence, CT had a sensitivity of 86%, a specificity of 98%, and an accuracy of 97%, whereas PET/CT had a sensitivity of 76%, a specificity of 98%, and an accuracy of 96%; these differences were not statistically significant (P = 0.089). For peritoneal carcinomatosis, CT had a sensitivity of 96%, a specificity of 100%, and an accuracy of 99%, whereas PET/CT had a sensitivity of 50%, a specificity of 100%, and an accuracy of 90%, a statistically significant difference (P = 0.001; Figure 2). For detecting locoregional, and lymph node recurrences, and liver metastasis, there were no statistically significant differences between CT and PET/CT (Table 3, Figures 3 and 4). For detecting bone metastasis, CT had a sensitivity of 20%, a specificity of 100%, and an accuracy of 97%, whereas PET/CT had a sensitivity of 100%, a specificity of 100%, and an accuracy of 100%, these differences were not statistically significant (P = 0.125; Figure 5).Two patients who had locoregional recurrence with inadequate bowel distension were missed on CT. Another two patients with locoregional recurrence were missed on PET/CT, and their recurrences were concealed by physiological uptake of remnant stomach. Two patients who had lymph node recurrence of less than 10 mm in the short axis were missed on CT, but were detected on PET/CT. Three patients who had lymph node recurrence without significant hypermetabolism were missed on PET/CT. One hepatic metastasis (about 1.5 cm) was not detected on CT and the metastatic tumor had no attenuation difference compared to the adjacent liver. One patient with peritoneal carcinomatosis was considered as having peritonitis on CT, because he also had a very large abdominal abscess. Twelve patients who had peritoneal carcinomatosis without significant hypermetabolism were missed on PET/CT.
Adenocarcinoma was the most common type of gastric carcinoma (Table 1). On the pathological type-based analysis, CT had a sensitivity of 98%, a specificity of 95%, and an accuracy of 96% for detecting recurrence of adenocarcinoma, whereas PET/CT had a sensitivity of 80%, a specificity of 95%, and an accuracy of 88%; these differences were not statistically significant (P = 0.035; Table 4). For detecting the recurrence of signet ring cell carcinoma and mucinous adenocarcinoma patients, CT and PET/CT had no significant differences.
| Type | Imaging | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) | P value |
| Overall | CT | 97 (58/60) | 97 (58/60) | 97 (58/60) | 97 (58/60) | 97 (114/120) | 0.096 |
| PET/CT | 82 (49/60) | 95 (57/60) | 94 (49/52) | 84 (57/68) | 88 (106/120) | ||
| Adenocarcinoma | CT | 98 (50/51) | 95 (52/55) | 94 (50/53) | 98 (52/53) | 96 (102/106) | 0.035 |
| PET/CT | 80 (41/51) | 95 (52/55) | 93 (41/44) | 84 (52/62) | 88 (93/106) | ||
| Signet ring cell carcinoma | CT | 100 (5/5) | 100 (2/2) | 100 (5/5) | 100 (2/2) | 100 (7/7) | 1 |
| PET/CT | 80 (4/5) | 100 (2/2) | 100 (4/4) | 67 (2/3) | 86 (6/7) | ||
| Mucinous adenocarcinoma | CT | 75 (3/4) | 100 (3/3) | 100 (3/3) | 75 (3/4) | 86 (6/7) | 1 |
| PET/CT | 100 (4/4) | 100 (3/3) | 100 (4/4) | 100 (3/3) | 100 (7/7) |
The recurrence rate of early gastric cancer after curative resection is very low and PET/CT is not commonly used for these patients. However advanced gastric cancer cases show a high recurrence rate. Contrast-enhanced abdominal CT and PET/CT are commonly used imaging methods, but the ideal method for early detection of gastric carcinoma recurrence remains controversial.
In terms of the patient characteristics, the pathological stages of the recurrence group were statistically significantly higher than those of the control group. In the recurrence group, the stages of 33 patients (55%) were Ia, none was Ia, and seven patients were IV. The median time interval between the contrast-enhanced CT and PET/CT was also statistically significantly longer in the recurrence group than in the control group. One of the inclusion criteria for our study was an interval shorter than 1 mo between the CT and PET/CT examination. Most of the patients in the control group were prescribed both CT and PET/CT around the same day for their regular follow ups. All patients underwent only CT, and were only sometimes prescribed both CT and PET/CT for regular follow up. However, when recurrence was suspected in a regular follow-up CT in the recurrence group, an additional PET/CT was prescribed to increase confidence or evaluate the recurrent lesion. Our institution is a tertiary referral hospital, and appointments for imaging studies were therefore delayed.
For detecting locoregional and lymph node re-currence, the diagnostic performance of CT and PET/CT was not significantly different in our study, which is in agreement with previous studies[7,13]. Locoregional recurrence manifests as localized bowel wall thickening of the anastomotic site or remnant stomach on CT. However, inadequate bowel distension, surgical plication, bowel adhesion, and stomal polypoid hypertrophic gastritis can lower the specificity of the CT[23]. In PET/CT, focal hypermetabolism of the anastomotic site or remnant stomach was considered locoregional recurrence; however, physiological uptake in the bowel can compromise the diagnostic performance of PET-CT[7]. These disadvantages of both contrast-enhanced CT and PET/CT contribute to decreased diagnostic performance in the detection of recurrent gastric cancer. In our study, some locoregional recurrences were missed on CT with inadequate bowel distension and some were missed on PET/CT with physiological uptake in the stomach.
In this study, PET/CT was more sensitive than CT in detecting hepatic metastasis (100% vs 66%), but the number of patients who had hepatic metastases was too small to show statistical significance. Kinkel et al[16] reported that PET was the most sensitive method for the detection of hepatic metastases from gastrointestinal-tract cancer. However, other studies showed no significant difference between CT and PET/CT in detecting hepatic metastases[7,13].
CT was more sensitive for peritoneal carcinomatosis compared with PET/CT in this study. Previous studies showed similar results[7,11,13]. The detection of peritoneal carcinomatosis is difficult in cross-sectional images because the lesions are usually small (below 1 cm) and often flat. On PET/CT, physiological peritoneal FDG uptake due to involuntary muscle activity during scanning can hide underlying peritoneal metastases[14]. Therefore, patients who suspected to have peritoneal carcinomatosis in contrast-enhanced abdominal CT may not see any benefit in any additional PET/CT due to its moderate sensitivity.
For bone metastasis, CT had a detection sensitivity of 20% compared to 100% for PET/CT in our study. However, this difference was not statistically significant. Previous research showed that, PET/CT was more sensitive in detecting bone metastasis than CT mostly because it provides functional information[24]. However, the number of patients with bone metastasis enrolled in this study was too small to show statistical significance.
In our study, CT was more sensitive than PET/CT in detecting the recurrence of adenocarcinoma, contrary to the findings of previous studies[7,25]. Considering the high proportion of patients with a pathological type of adenocarcinoma was 88.6% in this study, CT efficacy may be superior to that of PET/CT. There have been reports that FDG avidity depends on histological type. Mucinous adenocarcinoma and signet ring cell carcinoma have low FDG avidity[25]. Although the number of mucinous and signet right cell carcinoma cases was not large, there was no statistically significant difference in detecting recurrence.
This study had two limitations. The first was its retrospective study design; thus, the indications for PET/CT were not defined and it was not possible to standardize the methods of follow-up imaging studies. This could cause selection bias, because patients who had not undergone CT or PET/CT were excluded. Secondly, not all the recurrent lesions were diagnosed histopathologically. Deeply located lymph node metastasis is difficult to histopathologically confirm in clinical practice. Practically, however, a clinical follow-up is a useful diagnostic method to confirm recurrence. It was regarded as a negative lesion when there was no change between images obtained with at least a 6 mo interval, as in a previous study[13]. But 6 mo of imaging follow up may not be enough to confirm the absence of recurrence.
In conclusion, the overall diagnostic performance of contrast-enhanced CT and PET/CT in patient- and lesion-based analysis was not significantly different in our study. However, contrast-enhanced CT was superior to PET/CT in the detection of peritoneal carcinomatosis. In patients with the most common pathological type of adenocarcinoma, contrast-enhanced CT might be more sensitive in detecting recurrence than PET/CT. Contrast-enhanced CT could thus be the primary method of surveillance for the recurrence of gastric carcinoma after curative resection.
Gastric carcinoma is still a leading cause of cancer death worldwide. Surgical resection is the potentially curative treatment for gastric cancer. Contrast-enhanced abdominal computed tomography (CT) and positron emission tomography/computed tomography (PET/CT) are commonly used imaging methods for surveillance of recurrence after gastric cancer surgery. But ideal method for early detection of gastric carcinoma recurrence remains controversial.
Contrast-enhanced CT was superior to PET/CT in the detection of peritoneal carcinomatosis and pathologic type of adenocarcinoma. Contrast-enhanced CT could thus be the primary method of surveillance for the recurrence of gastric carcinoma after curative resection.
Contrast-enhanced abdominal CT and PET/CT are commonly used imaging methods for surveillance of recurrence after gastric cancer surgery. But there have been controversies about the role of CT, compared to PET/CT, in evaluating the recurrence of gastric carcinoma after curative surgery. We found contrast-enhanced CT was superior to PET/CT in the detection of peritoneal carcinomatosis and pathologic type of adenocarcinoma
Contrast-enhanced CT could be the primary method of surveillance for the recurrence of gastric carcinoma after curative resection.
This manuscript is very interesting and should be published in priority after minimal revision has been made. It is a retrospective study comparing the value of contrast enhanced abdominal CT and the fluorodeoxyglucose. Positron emission tomography/computed tomography for detecting recurrence of gastric carcinoma after curative resection.
Manuscript source: Unsolicited manuscript
P-Reviewer: Misiakos EP, Garcia-Olmo D S-Editor: Qi Y L-Editor: E-Editor:
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Misiakos EP, Garcia-Olmo D S- Editor: Qi Y
L- Editor: A E- Editor: Ma YJ
| 1. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9563] [Article Influence: 869.4] [Reference Citation Analysis (0)] |
| 2. | Park JM, Kim YH. Current approaches to gastric cancer in Korea. Gastrointest Cancer Res. 2008;2:137-144. [PubMed] |
| 3. | D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 486] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 4. | Markar SR, Karthikesalingam A, Jackson D, Hanna GB. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol. 2013;20:2328-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Lai JF, Xu WN, Noh SH, Lu WQ. Effect of World Health Organization (WHO) Histological Classification on Predicting Lymph Node Metastasis and Recurrence in Early Gastric Cancer. Med Sci Monit. 2016;22:3147-3153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Moriguchi S, Maehara Y, Korenaga D, Sugimachi K, Nose Y. Risk factors which predict pattern of recurrence after curative surgery for patients with advanced gastric cancer. Surg Oncol. 1992;1:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Kim DW, Park SA, Kim CG. Detecting the recurrence of gastric cancer after curative resection: comparison of FDG PET/CT and contrast-enhanced abdominal CT. J Korean Med Sci. 2011;26:875-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 8. | Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 361] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 9. | Lee SY, Lee JH, Hwang NC, Kim YH, Rhee PL, Kim JJ, Paik SW, Rhee JC, Sohn TS, Kim S. The role of follow-up endoscopy after total gastrectomy for gastric cancer. Eur J Surg Oncol. 2005;31:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Jadvar H, Tatlidil R, Garcia AA, Conti PS. Evaluation of recurrent gastric malignancy with [F-18]-FDG positron emission tomography. Clin Radiol. 2003;58:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Park MJ, Lee WJ, Lim HK, Park KW, Choi JY, Kim BT. Detecting recurrence of gastric cancer: the value of FDG PET/CT. Abdom Imaging. 2009;34:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Lee JW, Lee SM, Son MW, Lee MS. Diagnostic performance of FDG PET/CT for surveillance in asymptomatic gastric cancer patients after curative surgical resection. Eur J Nucl Med Mol Imaging. 2016;43:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, Im SA, Kim TY, Kim WH, Heo DS. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer. 2009;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, Maes A, Mortelmans L. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002;29:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Cayvarlı H, Bekiş R, Akman T, Altun D. The Role of 18F-FDG PET/CT in the Evaluation of Gastric Cancer Recurrence. Mol Imaging Radionucl Ther. 2014;23:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 363] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Baiocchi GL, Marrelli D, Verlato G, Morgagni P, Giacopuzzi S, Coniglio A, Marchet A, Rosa F, Capponi MG, Di Leo A. Follow-up after gastrectomy for cancer: an appraisal of the Italian research group for gastric cancer. Ann Surg Oncol. 2014;21:2005-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Ha HK, Kim HH, Kim HS, Lee MH, Kim KT, Shinn KS. Local recurrence after surgery for gastric carcinoma: CT findings. AJR Am J Roentgenol. 1993;161:975-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Lim JS, Yun MJ, Kim MJ, Hyung WJ, Park MS, Choi JY, Kim TS, Lee JD, Noh SH, Kim KW. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006;26:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Federle MP, Raman SP. Diagnostic Imaging: Gastrointestinal: Elsevier Health Sciences, 2015. Available from: https://www.elsevier.com/books/diagnostic-imaging-gastrointestinal/federle/978-0-323-37755-3. |
| 21. | Kim KA, Park CM, Park SW, Cha SH, Seol HY, Cha IH, Lee KY. CT findings in the abdomen and pelvis after gastric carcinoma resection. AJR Am J Roentgenol. 2002;179:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Kwak JY, Kim JS, Kim HJ, Ha HK, Yu CS, Kim JC. Diagnostic value of FDG-PET/CT for lymph node metastasis of colorectal cancer. World J Surg. 2012;36:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Kim KW, Choi BI, Han JK, Kim TK, Kim AY, Lee HJ, Kim YH, Choi JI, Do KH, Kim HC. Postoperative anatomic and pathologic findings at CT following gastrectomy. Radiographics. 2002;22:323-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Liu NB, Zhu L, Li MH, Sun XR, Hu M, Huo ZW, Xu WG, Yu JM. Diagnostic value of 18F-FDG PET/CT in comparison to bone scintigraphy, CT and 18F-FDG PET for the detection of bone metastasis. Asian Pac J Cancer Prev. 2013;14:3647-3652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, Lee JH, Ryu KW, Kim YW, Bae JM. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |