Copyright
©The Author(s) 2017.
World J Gastroenterol. Sep 7, 2017; 23(33): 6128-6136
Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6128
Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6128
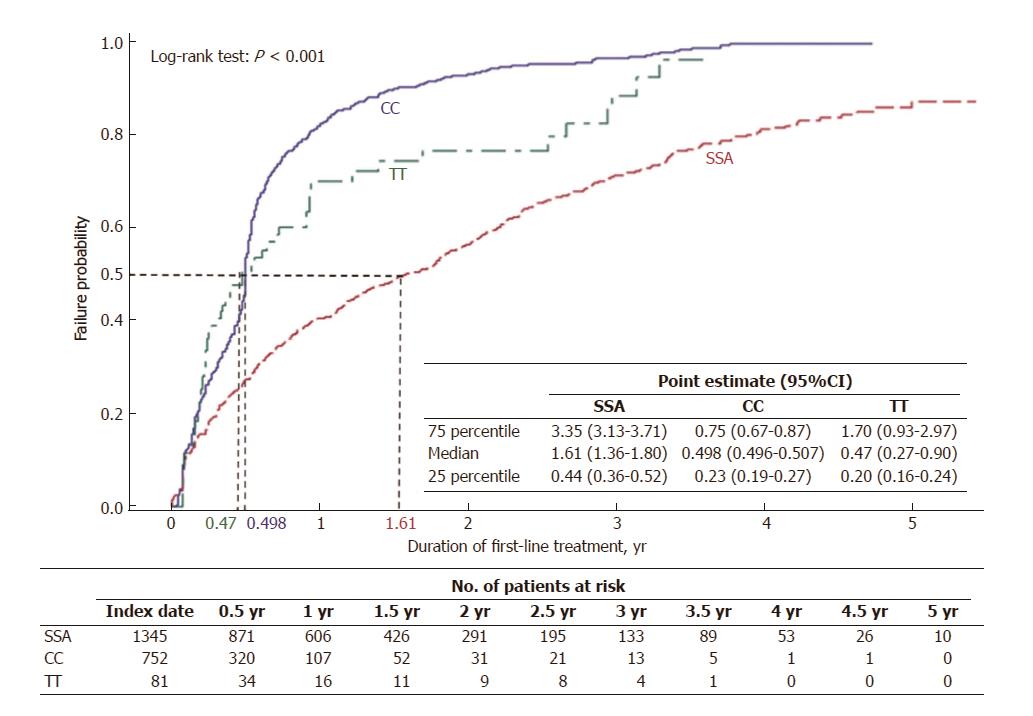
Figure 3 Time to discontinuation of first-line treatment.
By 588 d of treatment (1.61 yr), half of SSA initiators had discontinued treatment, compared to 182 d (0.498 yr) for half of CC users and 171 d (0.47 yr) for half of TT users to discontinue treatment. SSA: Somatostatin analogues; CC: Cytotoxic chemotherapy; TT: Targeted therapy.
- Citation: Benson III AB, Broder MS, Cai B, Chang E, Neary MP, Papoyan E. Real-world treatment patterns of gastrointestinal neuroendocrine tumors: A claims database analysis. World J Gastroenterol 2017; 23(33): 6128-6136
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6128