Copyright
©The Author(s) 2017.
World J Gastroenterol. Sep 7, 2017; 23(33): 6128-6136
Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6128
Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6128
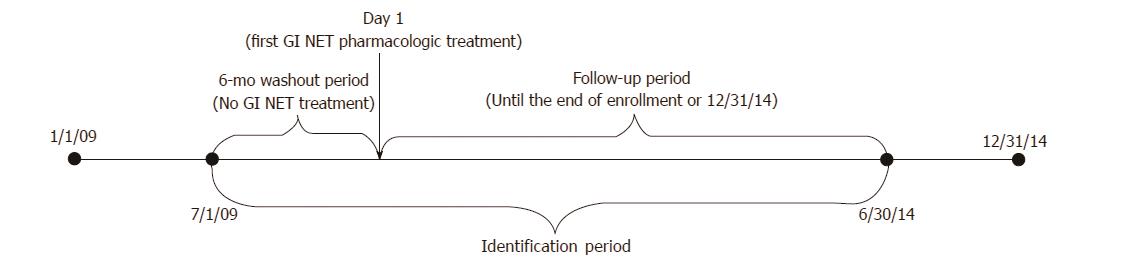
Figure 1 Study timeline.
The first gastrointestinal neuroendocrine tumors (GI NET) pharmacologic treatment claim on or after the appearance of the GI NET diagnosis code and within the ID period (7/1/2009 to 6/30/2014) was considered to be the index date. Patients were required to be enrolled for a baseline period of at least six months before the index date. Patient follow-up was variable and continued until the end of enrollment or the study end date (12/31/14), whichever was first.
- Citation: Benson III AB, Broder MS, Cai B, Chang E, Neary MP, Papoyan E. Real-world treatment patterns of gastrointestinal neuroendocrine tumors: A claims database analysis. World J Gastroenterol 2017; 23(33): 6128-6136
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6128