Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6111
Peer-review started: February 8, 2017
First decision: May 12, 2017
Revised: June 10, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: September 7, 2017
Processing time: 212 Days and 21.4 Hours
To investigate the role of calmodulin-dependent protein kinase II (CaMKII) in colon cancer growth, migration and invasion.
CaMKII expression in colon cancer and paracancerous tissues was evaluated via immunochemistry. Transcriptional and posttranscriptional levels of CaMKIIin tissue samples and MMP2, MMP9 and TIMP-1 expression in the human colon cancer cell line HCT116 were assessed by qRT-PCR and western blot. Cell proliferation was detected with the MTT assay. Cancer cell migration and invasion were investigated with the Transwell culture system and wound-healing assay.
We first demonstrated that CaMKII was over-expressed in human colon cancers and was associated with cancer differentiation. In the human colon cancer cell line HCT116, the CaMKII-specific inhibitor KN93, but not its inactive analogue KN92, decreased cancer cell proliferation. Furthermore, KN93 also significantly prohibited HCT116 cell migration and invasion. The specific inhibition of ERK1/2 or p38 decreased the proliferation and migration of colon cancer cells.
Our findings highlight CaMKII as a potential critical mediator in human colon tumor development and metastasis.
Core tip: In the present study, we demonstrated that calmodulin-dependent protein kinase II (CaMKII) was over-expressed in human colon cancers and was associated with cancer differentiation. We investigated the role of CaMKII in human colon cancer proliferation and migration. The results revealed that in the human colon cancer cell line HCT116, the CaMKII-specific inhibitor KN93, but not its inactive analogue KN92, decreased cancer cell proliferation. KN93 also significantly prohibited colon cancer cell migration and invasion. Additionally, we found that ERK1/2 and p38 were the targets of CaMKII regulation. These findings highlight CaMKII as a potential critical mediator in human colon tumor development and metastasis.
- Citation: Chen W, An P, Quan XJ, Zhang J, Zhou ZY, Zou LP, Luo HS. Ca2+/calmodulin-dependent protein kinase II regulates colon cancer proliferation and migration via ERK1/2 and p38 pathways. World J Gastroenterol 2017; 23(33): 6111-6118
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6111
Colorectal cancer (CRC) is the third most common cancer and the fourth leading cause of cancer death globally. Researchers estimate that roughly 1.4 million new cases of CRC will be diagnosed and 694000 deaths will result from CRC per year[1]. Despite improvements in multimodal anticancer strategies, the prognosis of advanced CRC is still poor, with 5-year survival rates for stage III and stage IV colon cancer at 65.4% and 12.8%, respectively[2]. A detailed understanding of the biological processes that modulate the progression and metastasis of CRC may provide new molecular pathogenesis targets for CRC diagnosis and benefit anti-tumor therapy.
The calcium ion (Ca2+) is a ubiquitous intracellular signal responsible for a broad range of cellular events, such as cell growth, cytoskeletal organization, regulation of synaptic transmission, and Ca2+ homeostasis[3]. The Ca2+/calmodulin (CaM)-dependent protein kinases (CaMKI, CaMKII and CaMKIV) are multifunctional serine/threonine kinases whose activity is activated upon Ca2+/CaM binding[4]. The upstream CaMK kinases (CaMKKs) phosphorylate a critical Thr 200 in the activation-loop to activate CaMKIV, whereas CaMKII is fully activated by the autophosphorylation of its own Thr 286[5]. Studies have revealed that the calcium signal is involved in cancer cell proliferation, invasion, tumor growth and metastasis[6-9]. However, fewer studies have focused on CaMKII, one of the most important sensors and regulators of the Ca2+ signal, in digestive cancers, especially in CRC.
In the present study, we first demonstrated that CaMKII was over-expressed in human colon cancers and was associated with cancer differentiation. We further sought to investigate the role of CaMKII in human colon cancer proliferation and migration. The results revealed that in the human colon cancer cell line HCT116, the CaMKII-specific inhibitor KN93, but not its inactive analogue KN92, decreased cancer cell proliferation. KN93 also significantly prohibits HCT116 cell migration and invasion. Additionally, we determined that CaMKII inhibition decreased the phosphorylation of ERK1/2 and p38. The specific inhibition of ERK1/2 or p38 decreased the proliferation and migration of colon cancer cells. These findings highlight CaMKII as a potential critical mediator in human colon tumor development and metastasis.
Antibodies against CaMKIIα, ERK1/2, phospho (p)-ERK1/2, p38, p-p38, MMP2, MMP9, TIMP-1 and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). KN-93 (2-[N-(2-Hydroxyethyl)]-N-(4-methoxybenzene-sulfonyl)] amino-N-(4- chlorocinnamyl)-N-methylbenzylamine), KN-92 (2-[N-(4-Methoxybenzenesulfonyl)] amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), PD98059 and SB203580 were obtained from Calbiochem (La Jolla, CA, United States). Other chemicals of the highest purity were purchased from Sigma (St. Louis, MO, United States).
Colon tissue samples were obtained from the Department of Gastrointestinal Surgery at Renmin Hospital of Wuhan University in accordance with local ethics committees. All samples were obtained from the primary colon sites of pretreatment cases of paracancerous lesions (n = 5), well-differentiated colon cancer tissues (n = 6) and poorly differentiated colon cancer tissues (n = 6). All samples were analyzed and assessed by two histological specialists working blindly.
The human colon cancer cell line HCT116 was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Rockville, MD, United States) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin (Life Technologies, Rockville, MD, United States) at 37 °C in a humidified atmosphere with 5% CO2. For conditioned media, 3 × 105 HCT116 cells were seeded in 6-well plates and then stimulated for the indicated amount of time.
Immunohistochemistry (IHC) for CaMKII was performed on paraffin-embedded tissues from paracancerous tissues, well-differentiated colon cancers and poorly differentiated colon cancers. Specimens were fixed in a 4% formaldehyde solution for 12-48 h and embedded in paraffin. IHC was performed on 3.5 μm tissue sections on slides. Heat-induced epitope retrieval was carried out. For immunofluorescent staining for CaMKII, all slides were blocked with serum solution and incubated with the primary antibody overnight at 4 °C. After rinsing again, the slides were incubated for 45 min at room temperature with secondary antibodies. Visualization was achieved with the DAB (3,3'-diaminobenzidine) detection kit, and the cell nuclei were counterstained using hematoxylin. After staining, five fields (at a magnification of × 100) were randomly selected in each slide. Staining was quantified with Image J software and evaluated by two investigators following the “blinded” principle.
Cell proliferation was measured with the MTT assay (ATCC, Manassas, VA, United States). A total of 5 × 103 cells were seeded at the well bottoms of 96-well culture plates and treated with TGFβ1 or conditioned medium from fibroblasts or colon cancer cells in triplicate. After treatment, MTT solution [3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazoliumbromide] was added to the culture medium (0.5 mmol/L), and plates were incubated for 2 h at 37 °C with 5% CO2. Detergent solution was then added to solubilize formazan crystals. Finally, the optical density was determined at 540 nm with a Benchmark Plus microplate reader (Bio-Rad, Hercules, CA, United States).
Protein from unstained paraffin-embedded samples was deparaffinized and rehydrated via successive washes in xylene/ethanol and water. After incubation in extraction buffer at 100 °C for 20 min followed by a 2 h incubation at 80 °C, proteins were obtained by centrifugation. Whole cell lysates were prepared with RIPA lysis buffer containing protease and phosphatase inhibitor cocktail. Protein concentrations of cell lysates were determined with the bicinchoninic acid protein assay. A total of 30-40 μg of lysate were loaded onto a 10% SDS-PAGE gel and subjected to gel electrophoresis. Resolved proteins were transferred to a polyvinylidine fluoride membrane. Membranes were then blocked in 5% non-fat dry milk in Tris-buffered saline with Tween (TBS-T) for 1 h and then incubated with antibodies to GAPDH, CaMKIIα (Cell Signaling Technology), ERK1/2, phospho-ERK1/2, p38, p-p38, MMP2, MMP9 and TIMP-1 at 4 °C overnight. Membranes were washed with TBS-T and then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The blotting bands were visualized with enhanced chemiluminescence. GAPDH was used as a loading control.
Cells were seeded at 106 cells per 10 cm dish and were allowed to grow to 80% confluency in complete media. Cells were removed with 0.025% ethylenediaminetetraacetic acid and centrifuged for 5 min at 1100 rpm. Cell pellets were resuspended in 1 mL of TRIzol (Life Technologies, Carlsbad, CA, United States), and RNA was extracted according to the manufacturer’s protocol. Total RNA in unstained paraffin-embedded samples was extracted with the RNeasy FFPE Kit (Qiagen, Hilden, Germany). The SuperScript First-Strand Kit (Life Technologies) was used to synthesize cDNA from 5 μg of total RNA. Quantitative PCR was established with the RT2 SYBR Green Flour FAST Mastermix (Qiagen, Venlo, the Netherlands) and run on a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad). Data were analyzed with Bio-Rad CFX Manager 3.0 software and are shown as relative fold-changes.
For all PCR reactions, GAPDH was used as an endogenous control, and CT values were normalized to levels of GAPDH expression. Primers were designed with Integrated DNA Technologies PrimerQuest software (Coralville, IA, United States). Sequences used to analyze RNA expression include the following: CaMKII, Forward: 5’-GAG AGC ACC AAC ACC ACC ATC G-3’ and Reverse: 5’-AGG CTG ACT CGT CGC CCA TCA GG-3’; MMP2, Forward: 5’-TCT CCT GAC ATT GAC CTT GGC-3’ and Reverse: 5’-CAA GGT GCT GGC TGA GTA GAT C-3’; MMP9, Forward: 5’-TTG ACA GCG ACA AGA AGT GG-3’ and Reverse: 5’-GCC ATT CAC GTC GTC CTT AT-3’; TIMP-1, Forward: 5’-CTT CTG GCA TCC TGT TGT TG-3’ and Reverse: 5’-GGT ATA AGG TGG TCT GGT TG-3’; GAPDH, Forward: 5’-ACA GTC CAT GCC ATC ACT GCC-3’ and Reverse: 5’-GCC TGC TTC ACC ACC TTC TTG-3’. The results are averaged from three independent experiments.
A 24-well Transwell plate (Fisher Scientific, Hampton, NH, United States) was used to measure migratory and invasive ability in vitro. A total of 2 × 105 HCT-116 cells were plated on the bottom of each well. For the migration assay, 1 × 105 colon cancer cells were plated in the top chamber with a non-coated membrane. For the invasion assay, 2 × 105 HCT-116 cells were seeded in the top chamber coated with Matrigel (BD Biosciences, San Jose, CA, United States). In both assays, cells were cultured in DMEM containing 10% FBS with the indicated treatment. After incubation at 37 °C for 24 h, migrated cells were fixed and stained with the Diff-Quik stain and counted in four random fields. The experiments were performed in triplicate wells, and each experiment was performed at least two or three times as indicated.
A wound-healing assay was performed with 6-well plates. HCT116 cells were seeded at confluency 24 h before the experiment. The cell layers were carefully scratched with 200-mL sterile pipette tips and washed twice with fresh medium. The cells were photographed under a light microscope at × 200 magnification 24 h later.
All experiments were performed in triplicate. Data are expressed as the mean ± SD. Differences between groups were calculated via one-way analysis of variance. Differences were considered significant at P < 0.05.
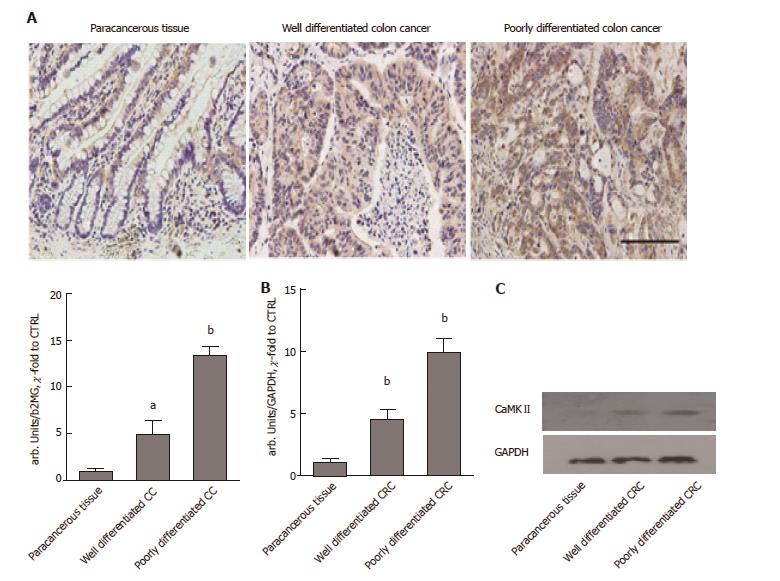
The expression of CaMKII was evaluated by IHC with a monoclonal CaMKII antibody on sections obtained from formalin-fixed, paraffin-embedded samples of paracancerous tissue, well-differentiated colon cancer samples and poorly differentiated colon cancer samples. As expected, CaMKII was significantly overexpressed in the poorly differentiated colon cancer samples and well-differentiated cancer samples compared to the paracancerous tissues (Figure 1A). A quantification assay suggested that the expression of CaMKII was highest in poorly differentiated colon cancer tissues. We further evaluated CaMKII protein and mRNA levels in cancer and paracancerous tissues. The results were similar to the findings of the above studies (Figure 1B and C). We deduced that CaMKII may be a potential regulator in colon cancer development.
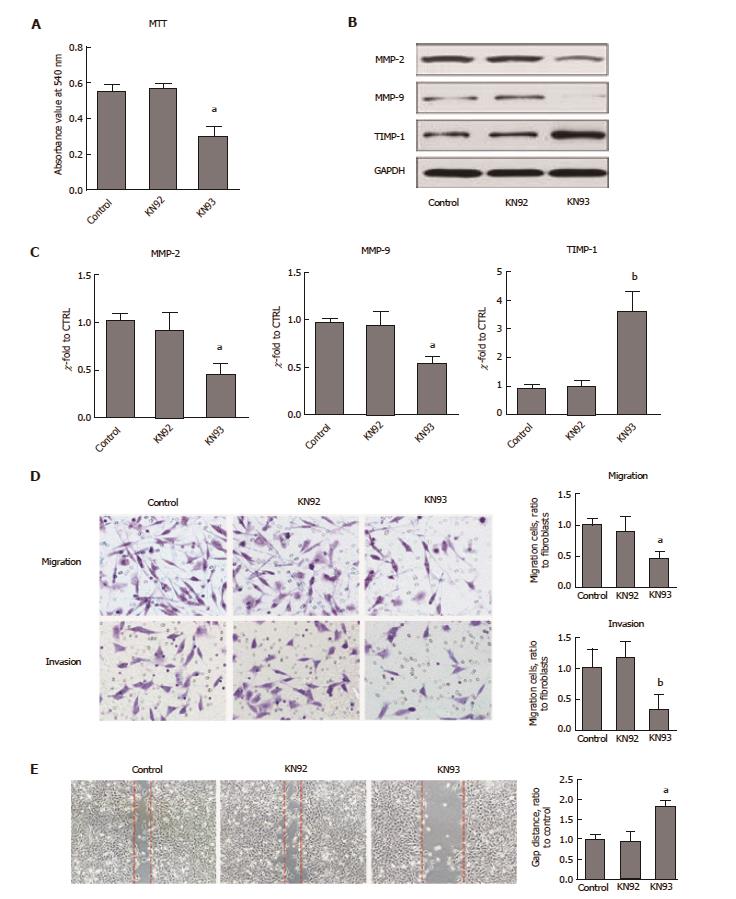
CaMKII has been shown to drive cancer growth and metastasis. To ascertain whether CaMKII regulates colon cancer proliferation and migration, the CaMKII specific inhibitor KN93 was selected. Human colon cancer HCT116 cells were treated with KN93, and its inactive analogue, KN92, was used as an additional control. We found that KN93 inhibited colon cancer cell proliferation (Figure 2A). Importantly, in migration and invasion experiments, the motility of HCT116 cells was significantly attenuated (Figure 2B and C). Furthermore, CaMKII inhibition decreased MMP2 and MMP9 expression at both transcriptional and posttranscriptional levels. The opposite results were detected for TIMP-1 expression (Figure 2D and E). Thus, we concluded that CaMKII is required for colon cancer cell proliferation and migration.
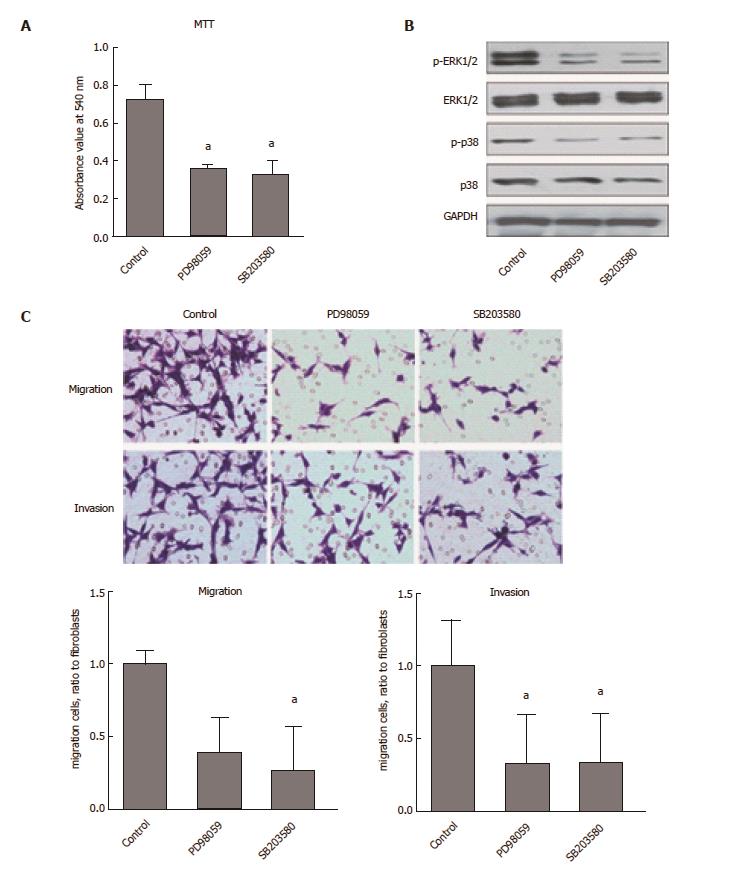
We further investigated the molecular mechanism of CaMKII regulation in human colon cancer cells. As shown in Figure 3, KN93, but not KN92, decreased the phosphorylation of ERK1/2 and p38, which are involved in colon cancer development. Pretreatment with specific inhibitors of ERK1/2 or p38 (PD98059, SB203580) decreased HCT116 cell proliferation, migration and invasion. These data demonstrated that the effects of CaMKII on human colon cancer are ERK1/2- and p38-dependent.
Taken together, these results indicate that CaMKII is an important regulator of human colon cancer growth and migration.
It is widely accepted that intracellular Ca2+ signaling pathways regulate various biological events, including cell proliferation, motility, activation and differentiation, during cancer development[10,11]. In the present study, we demonstrated that CaMKII is over-expressed in colon cancers and highly expressed in poorly differentiated colon tumors. We highlight the role of CaMKII in colon cancer growth and metastasis.
CaMKII inhibitors can inhibit CaMKII activity by interacting with the Ca2+/CaM-binding site or interfering with its catalytic activities. Previous studies revealed that CaMKII-specific inhibitors such as KN-62, KN-93, and autocamtide 2-related inhibitory peptide inhibit CaMKII-dependent processes in tumor and normal cells, causing cell cycle arrest, cellular apoptosis, or the inhibition of cell proliferation[12-14].
Some studies have implicated CaMKII as an important player in cancer cell proliferation. CaMKII inhibition decreased ovarian cancer cell motility and decreased tumor growth and metastasis[15]. The in vivo administration of KN-93 to mice xenografted with human osteosarcoma cells significantly decreased intratibial and subcutaneous tumor growth[16]. In CRC, we demonstrated that CaMKII inhibition strongly inhibited human colon cancer cell proliferation (Figure 2A).
Recent studies support the important role of CaMKII in cancer invasion and metastasis. The up-regulation of CaMKIIα was found in primary osteosarcoma tissues from patients and in aggressive osteosarcoma cell lines[17]. CaMKIIα depletion decreased motility and invasion, whereas CaMKIIα over-expression increased the tumorigenic properties of osteosarcoma cells in vitro. Our current results revealed the important role of CaMKII in colon cancer migration and invasion. KN93 dramatically decreased the expression of MMP2 and MMP9, increased TIMP-1 levels and prohibited the invasive motility of colon cancer cells (Figure 2). CaMKII is a critical regulator of colon cancer progression.
The MAPK pathway is one of the important downstream targets in CaMKII regulation[4]. CaMKII directly or indirectly up-regulated multiple signaling pathways, such as ERK1/2, AKT1 and β-catenin, and is involved in regulating the survival and proliferation of non-small cell lung cancer cells[6]. We further investigated whether ERK1/2 and p38 were involved in CaMKII pathways. Our data showed that the inhibition of CaMKII by KN93 significantly decreased ERK1/2 and p38 phosphorylation (Figure 3). The specific inhibition of ERK1/2 or p38 decreased the proliferation, migration and invasion of HCT116 cells. Therefore, CaMKII mediates colon cancer growth and migration via ERK1/2- and p38-dependent pathways.
Taken together, the current study provided evidence for CaMKII as a pivotal regulator of colon cancer cell proliferation and migration. CaMKII is a potentially important target in CRC treatment.
Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) is one of the most important sensors and regulators of the Ca2+ signal which is involved in cancer cell proliferation, invasion, tumor growth and metastasis. Despite the critical role of CaMKII, fewer studies focus on it in digestive cancers, especially in colorectal cancer.
Recently, studies have revealed CaMKII is an effective controller in ovarian cancer and osteosarcoma cell motility, cell growth and metastasis.
In the present study, the authors demonstrated that CaMKII was over-expressed in human colon cancers and was associated with cancer differentiation. Meanwhile, in the human colon cancer cell line HCT116, the CaMKII-specific inhibitor KN93, but not its inactive analogue KN92, decreased cancer cell proliferation. CaMKII inhibition also significantly prohibited colon cancer cell migration and invasion. Additionally, ERK1/2 and p38 were the targets of CaMKII regulation.
These findings highlight CaMKII as a potential critical mediator in human colon tumor development and metastasis. Its relationship to colon cancer differentiation indicates CaMKII a potential target for clinical diagnosis and prognosis.
CaMKII is a ubiquitous serine/threonine protein kinase. Autophosphorylation of CaMKII at threonine 286 switches the kinase from Ca2+-dependent to Ca2+-independent activity and leads to sustained CaMKII auto-activation. More importantly, this activation is thought to phosphorylate numerous different proteins and regulate diverse signaling pathways.
This is a brief study concerning the role of Ca2+/calmodulin-dependent protein kinase II involved in invasion and migration of the colon cancer. The study is interesting and topical. The experiments were well designed. The manuscript is clear and structured very well.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, b
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pan HC, Perse M S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Xu XR
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20369] [Article Influence: 2036.9] [Reference Citation Analysis (19)] |
| 2. | Lan YT, Yang SH, Chang SC, Liang WY, Li AF, Wang HS, Jiang JK, Chen WS, Lin TC, Lin JK. Analysis of the seventh edition of American Joint Committee on colon cancer staging. Int J Colorectal Dis. 2012;27:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Leung CS, Yeung TL, Yip KP, Pradeep S, Balasubramanian L, Liu J, Wong KK, Mangala LS, Armaiz-Pena GN, Lopez-Berestein G. Calcium-dependent FAK/CREB/TNNC1 signalling mediates the effect of stromal MFAP5 on ovarian cancer metastatic potential. Nat Commun. 2014;5:5092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Wang YY, Zhao R, Zhe H. The emerging role of CaMKII in cancer. Oncotarget. 2015;6:11725-11734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 376] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Chai S, Qian Y, Tang J, Liang Z, Zhang M, Si J, Li X, Huang W, Xu R, Wang K. RETRACTED: Ca(2+)/calmodulin-dependent protein kinase IIγ, a critical mediator of the NF-κB network, is a novel therapeutic target in non-small cell lung cancer. Cancer Lett. 2014;344:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S, Rebhan M, Raman P, Guy CT, Wetzel K. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA. 2013;110:E1026-E1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Wang T, Guo S, Liu Z, Wu L, Li M, Yang J, Chen R, Liu X, Xu H, Cai S. CAMK2N1 inhibits prostate cancer progression through androgen receptor-dependent signaling. Oncotarget. 2014;5:10293-10306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Wang C, Li N, Liu X, Zheng Y, Cao X. A novel endogenous human CaMKII inhibitory protein suppresses tumor growth by inducing cell cycle arrest via p27 stabilization. J Biol Chem. 2008;283:11565-11574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem. 2006;97:56-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026-14040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | An P, Tian Y, Chen M, Luo H. Ca(2+) /calmodulin- dependent protein kinase II mediates transforming growth factor-β-induced hepatic stellate cells proliferation but not in collagen α1(I) production. Hepatol Res. 2012;42:806-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Eleftheriadis T, Pissas G, Karioti A, Antoniadi G, Golfinopoulos S, Liakopoulos V, Mamara A, Speletas M, Koukoulis G, Stefanidis I. Uric acid induces caspase-1 activation, IL-1β secretion and P2X7 receptor dependent proliferation in primary human lymphocytes. Hippokratia. 2013;17:141-145. [PubMed] |
| 14. | An P, Zhu JY, Yang Y, Lv P, Tian YH, Chen MK, Luo HS. KN-93, a specific inhibitor of CaMKII inhibits human hepatic stellate cell proliferation in vitro. World J Gastroenterol. 2007;13:1445-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Ma S, Yang Y, Wang C, Hui N, Gu L, Zhong H, Cai Z, Wang Q, Zhang Q, Li N. Endogenous human CaMKII inhibitory protein suppresses tumor growth by inducing cell cycle arrest and apoptosis through down-regulation of the phosphatidylinositide 3-kinase/Akt/HDM2 pathway. J Biol Chem. 2009;284:24773-24782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Yuan K, Chung LW, Siegal GP, Zayzafoon M. alpha-CaMKII controls the growth of human osteosarcoma by regulating cell cycle progression. Lab Invest. 2007;87:938-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Daft PG, Yuan K, Warram JM, Klein MJ, Siegal GP, Zayzafoon M. Alpha-CaMKII plays a critical role in determining the aggressive behavior of human osteosarcoma. Mol Cancer Res. 2013;11:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |