Copyright
©The Author(s) 2017.
World J Gastroenterol. Aug 28, 2017; 23(32): 5925-5935
Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5925
Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5925
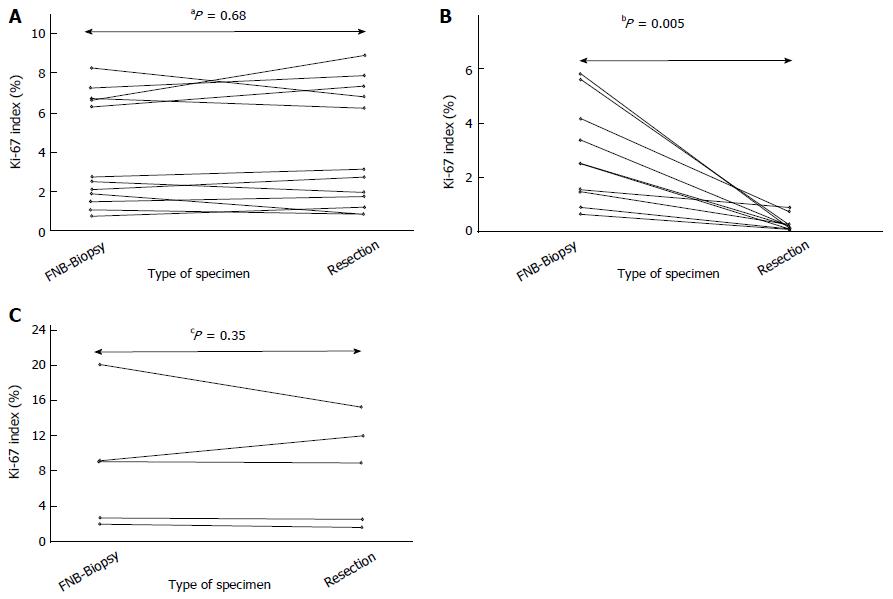
Figure 3 The Ki-67-index (%) of the fine-needle biopsy and of the corresponding resected specimen in each patient who underwent resection.
A: Patients not treated with neoadjuvant imatinib; B: Patients treated with neoadjuvant imatinib who carried an imatinib-sensitizing KIT- or PDGFRA-mutation; C: Patients treated with neoadjuvant imatinib who carried a KIT- or PDGFRA-mutation (or wild type profile), which indicates resistance to imatinib.
- Citation: Hedenström P, Nilsson B, Demir A, Andersson C, Enlund F, Nilsson O, Sadik R. Characterizing gastrointestinal stromal tumors and evaluating neoadjuvant imatinib by sequencing of endoscopic ultrasound-biopsies. World J Gastroenterol 2017; 23(32): 5925-5935
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5925