Copyright
©The Author(s) 2017.
World J Gastroenterol. Aug 14, 2017; 23(30): 5589-5601
Published online Aug 14, 2017. doi: 10.3748/wjg.v23.i30.5589
Published online Aug 14, 2017. doi: 10.3748/wjg.v23.i30.5589
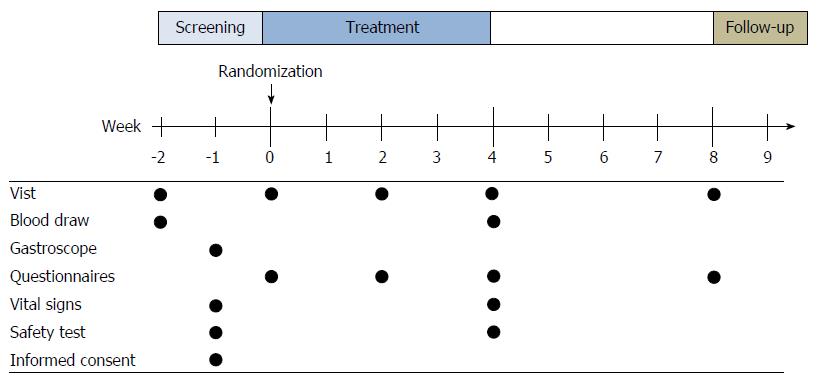
Figure 1 Study design.
There was a screening period of 2 wk before randomization. Blood draw, gastroscopy, and physical examination were performed and written informed consent were obtained from all patients in this period. Study visits were arranged at -2 wk, 0 wk, 2 wk, 4 wk and 8 wk. Some questionnaires were performed by phone during the treatment period, and this was followed by a 4-wk follow-up period.
- Citation: Lv L, Wang FY, Ma XX, Li ZH, Huang SP, Shi ZH, Ji HJ, Bian LQ, Zhang BH, Chen T, Yin XL, Tang XD. Efficacy and safety of Xiangsha Liujunzi granules for functional dyspepsia: A multi-center randomized double-blind placebo-controlled clinical study. World J Gastroenterol 2017; 23(30): 5589-5601
- URL: https://www.wjgnet.com/1007-9327/full/v23/i30/5589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i30.5589