Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5412
Peer-review started: March 29, 2017
First decision: April 26, 2017
Revised: May 9, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: August 7, 2017
Processing time: 133 Days and 3.9 Hours
To assess the prevalence of a panel of serologic markers that reflect gut barrier dysfunction in a mixed cohort of pediatric and adult primary sclerosing cholangitis (PSC) patients.
Sera of 67 PSC patients [median age (range): 32 (5-79) years, concomitant IBD: 67% and cirrhosis: 20%] were assayed for the presence of antibodies against to F-actin (AAA IgA/IgG) and gliadin (AGA IgA/IgG)] and for serum level of intestinal fatty acid-binding protein (I-FABP) by ELISA. Markers of lipopolysaccharide (LPS) exposure [LPS binding protein (LBP)] and various anti-microbial antibodies [anti-OMP Plus IgA and endotoxin core IgA antibody (EndoCAb)] were also determined. Poor disease outcome was defined as orthotopic liver transplantation and/or liver-related death during the follow-up [median: 99 (14-106) mo]. One hundred and fifty-three healthy subjects (HCONT) and 172 ulcerative colitis (UC) patients were the controls.
A total of 28.4%, 28.0%, 9% and 20.9% of PSC patients were positive for AAA IgA, AAA IgG, AGA IgA and AGA IgG, respectively. Frequencies of AAA IgA and AAA IgG (P < 0.001, for both) and AGA IgG (P = 0.01, for both) but not AGA IgA were significantly higher compared to both of the HCONT and the UC groups. In survival analysis, AAA IgA-positivity was revealed as an independent predictor of poor disease outcome after adjusting either for the presence of cirrhosis [HR = 5.15 (1.27-20.86), P = 0.022 or for the Mayo risk score (HR = 4.24 (0.99-18.21), P = 0.052]. AAA IgA-positivity was significantly associated with higher frequency of anti-microbial antibodies (P < 0.001 for EndoCab IgA and P = 0.012 for anti-OMP Plus IgA) and higher level of the enterocyte damage marker (median I-FABPAAA IgA posvsneg: 365 vs 166 pg/mL, P = 0.011), but not with serum LBP level.
Presence of IgA type AAA identified PSC patients with progressive disease. Moreover, it is associated with enhanced mucosal immune response to various microbial antigens and enterocyte damage further highlighting the importance of the gut-liver interaction in PSC.
Core tip: Importance of gut-liver interaction in the pathogenesis of primary sclerosing cholangitis (PSC) has been certified by a variety of clinical and experimental evidences. Clinical manifestations and progression of the disease are heterogeneous. No reliable biomarkers have been identified to this point that is able to predict the pace of progression. In the present, prospective follow-up study, we evaluated the clinical importance of novel serological markers related to gut barrier function in the prediction of progressive disease course in a cohort of PSC patients. IgA type anti-F-actin antibody was identified as a novel serologic marker during the prognostic work-up of PSC. Presence of anti-F-actin IgA identified PSC patients with progressive disease course and was associated with enhanced mucosal immune response to various microbial antigens and enterocyte damage further highlighting the importance of the gut-liver interaction in PSC.
- Citation: Tornai T, Palyu E, Vitalis Z, Tornai I, Tornai D, Antal-Szalmas P, Norman GL, Shums Z, Veres G, Dezsofi A, Par G, Par A, Orosz P, Szalay F, Lakatos PL, Papp M. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J Gastroenterol 2017; 23(29): 5412-5421
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5412.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5412
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by persistent, progressive biliary inflammation, fibrosis and eventually end-stage liver disease. Clinical manifestations and progression of the disease are heterogeneous. No reliable biomarkers have been identified to this point that able to predict the pace of progression. Consequently, patients with PSC cannot be stratified properly[1].
The etiology of PSC is poorly understood. Importance of gut-liver interaction in the pathogenesis of the disease however, has been certified by a large body of clinical evidence[2]. On the one hand, there is a close relation of the disease with inflammatory bowel diseases (IBD), up to 80% of the patients have both conditions[3]. On the other hand, in PSC patients with concomitant IBD, high peritransplant IBD activity was associated with higher[4], while colectomy before liver transplantation with considerably lower recurrent rate of PSC[5]. Experimental findings also supported the role of inflamed permeable gut in the subsequent inflammation of the biliary tract[6,7]. Patients with PSC display an exaggerated immune response to intestinal endotoxins with a lack of tolerance to repeated endotoxin exposure[8,9]. Prolonged and active intestinal inflammation interrupts the gut mucosal barrier function with a subsequent endotoxin exposure to cholangiocytes. Disruption of cholangiocytes’ tight junctions expose them various substances, such as bile acids, that could promote injury and inflammation[3]. Disruption of cholangiocytes’ tight junctions is an important step in the development of PSC in animal models[10,11].
Recently, reliable biomarkers of gut barrier function have been identified. Concerning enterocyte integrity, intestinal fatty acid-binding protein (I-FABP), a cytoplasmic protein of enterocytes, was reported as marker of enterocyte damage that could be considered as the “troponin of the gut”[12]. Anti-F-actin IgA antibodies (AAA-IgA) directed against intracellular cytoskeletal actin filaments and anti-gliadin IgA antibodies (AGA-IgA) serve as the markers of the structural intestinal mucosal damage. Presence of AAA-IgA strongly correlated with the histological findings of total or subtotal small intestinal atrophy in patients with celiac disease[13]. Presence of AGA-IgA was associated with increased intestinal permeability in patients with cirrhosis and significant portal hypertension[14].
The aims of this study were to assess: (1) the prevalence of a panel of serologic markers that reflect gut barrier dysfunction in a mixed cohort of pediatric and adult PSC patients; (2) associations between these markers and both the clinical and laboratory characteristics of the disease; (3) whether serologic markers of gut failure is associated with the long-term disease course in PSC; and (4) association of gut failure markers with the markers of lipopolysaccharide exposure and mucosal immune reactivity.
We performed an observational cohort study among adult and pediatric PSC patients recruited in Hungarian referral hepatology centers (Hungarian Autoimmune Liver Disease Study Group). In total 67, well-characterized PSC patients with a complete clinical follow-up [adult: 56 (male/female: 40/16), median age at presentation: 29 years (IQR: 19-37), median disease duration: 6 years (3-12) and children: 11 (male/female: 8/3), median age at presentation: 10 years (IQR: 6-12), disease duration: 5 (1-7)] were included between January, 2006 and December, 2007. Clinical characteristics of patients at enrolment are presented in Table 1.
| Patients with PSC (n = 67) | |
| Age at diagnosis (yr) | 25 (17-36) |
| Disease duration (yr) | 6 (3-10) |
| Children | 10 (14.5) |
| Male | 49 (71.0) |
| Cirrhosis | 14 (20.3) |
| Prior OLTx | 4 (5.8) |
| IBD | 52 (75.4) |
| Crohn's Disease | 17 (32.7) |
| Ulcerative Colitis | 35 (67.3) |
| Overlap syndrome | 9 (13.0) |
| Small duct PSC | 5 (7.2) |
| Celiac disease | 1 (1.5) |
| Albumin (g/L) | 44 (40-47) |
| Bilirubin (μmol/L) | 15 (11-22) |
| ALT (U/L) | 52 (26-99) |
| AST (U/L) | 41 (28–66) |
| GGT (U/L) | 152 (60-310) |
| ALP (U/L) | 524 (307-822) |
| INR | 1 (1.0-1.2) |
| Platelet (109/L) | 252 (173-319) |
| Mayo risk score | -0.595 (-1.194-0.102) |
| Atypical P-ANCA IgG | 57 (85.1) |
| EMA IgA | 1 (1.5) |
| EMA IgG | 1 (1.5) |
Diagnosis of PSC was based on clinical, biochemical, serological and cholangiographic (magnetic resonance or endoscopic imaging) features or, when indicated, on histological findings[15]. Patients with any concomitant malignant disease were excluded. Blood samples and detailed description of clinical phenotypes were obtained at inclusion. Clinical data were determined by thorough review of patients’ medical records, which had been collected in a uniform format. Medical records that documented disease phenotype (age at onset, duration, type of PSC - large duct or small duct), presence and type of concomitant IBD, presence of overlap syndrome, presence of cirrhosis and portal hypertension related complications (e.g., ascites, encephalopathy, oesophageal varices or variceal bleeding), prior orthotopic liver transplantation (OLTx), co-morbidities and medication (e.g., ursodeoxycholic acid, steroid, immunosuppressive and/or biological therapy) at inclusion were retrospectively analyzed for the period prior to the prospective follow-up. At enrolment, revised Mayo risk score was calculated[16] and biochemical analyses were performed using standard routine laboratory protocols for tests including platelet count, creatinine, total bilirubin, albumin, international normalized ratio (INR) of prothrombin time, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and γ-glutamyl-transferase (GGT).
Fifty-five out of 67 PSC patients were available to be enrolled into a prospective follow-up study, where the treating physicians registered laboratory data, imaging and endoscopic findings, medical treatment, date and type of complications (cirrhosis, colorectal cancer, biliary tract cancer: cholangiocarcinoma gallbladder cancer or cholangitis) during regular and additional outpatient follow-up visits and inpatient stays. In Hungary, a follow-up visit is usually scheduled for every 6 mo at a specialized hepatology center (the actual interval varies between 3 mo-6 mo). Collected data were transferred and stored in a database for analysis. On December 1, 2015, all patients’ charts and data were reviewed and updated for the data points mentioned above. Adverse outcome was defined as need for OLTx and/or liver-related death (composite end-point). Follow-up for a particular patient was terminated if there was no further record available or adverse outcome occurred. Cases with non-liver related death were censored at the time of event. Median follow-up from inclusion was 2646 (IQR: 401-3130) d.
Healthy controls and patients with ulcerative colitis (UC) as disease control group were included.
Healthy control group consisted of 153 individuals selected from consecutive blood donors in Debrecen. Control subjects did not have any known gastrointestinal or liver diseases.
Ulcerative colitis group consisted of a previously reported cohort of 172 patients without PSC [male/female: 79/93, age: 34 (23-44) years][17].
Blood samples were obtained at enrolment from each patient and were frozen at -70 °C until testing. All the serological assays were performed in a blinded fashion without prior knowledge of the patient’s clinical information. Commercially available ELISAs were used according to the manufacturer’s protocol to determine serologic markers of gut barrier function, lipopolysaccharide (LPS) exposure and anti-microbial antibodies. Positive cut-off levels for individual markers were defined either by the manufacturer’s recommendation or by the 95th percentile of healthy controls and used to dichotomize absolute values.
Presence of IgA or IgG type antibodies against F-actin (AAA) and gliadin (AGA) (QUANTA Lite®; INOVA Diagnostics, San Diego, CA) and level of intestinal fatty acid-binding protein (I-FABP) (Hycult Biotechnology, Uden, Netherlands) were determined as the serologic markers of gut barrier function. The cut-off for positivity was 35 U and 25 U for both types of AAA and AGA, respectively.
Level of acute phase protein, lipopolysaccharide (LPS) - binding protein (LBP) and endotoxin core IgA antibody (EndoCAb IgA) (Hycult Biotechnology, Uden, Netherlands) were determined as markers of LPS exposure. The cut-off for positivity was 195 U/mL for EndoCAb IgA.
Of the classic anti-microbial serologic antibodies, anti-OMP Plus antibody (QUANTA Lite®, Inova Diagnostics, San Diego, CA, United States) was detected. This assay detects IgA antibody against multiple bacterial proteins derived from two species of intestinal bacteria (one Gram-positive and one Gram-negative). Neither bacteria are from the phylum Proteobacteria, of which Escherichia coli is a member. The cut-off for positivity was 25 U as recommended by the manufacturer.
Atypical P-ANCA and anti-endomysial (EMA) antibodies were determined by commercially available indirect immunofluorescence test (IIF) as a part of the differential diagnostic procedure of autoimmune liver diseases and celiac disease. Atypical P-ANCA (IgA and IgG) and EMA (IgA and IgG) antibody positivities were considered in case of specific fluorescencent patterns in IIFT (Euroimmun Medizinische Labordiagnostika AG, Lübeck, Germany) at a dilution ≥ 1:32 or 1:10, respectively as recommended by the manufacturer. Detailed description has been reported previously[17,18].
The study protocol was approved by the Regional and Institutional Research Ethics Committee of University of Debrecen and the National Scientific and Research Ethics Committee [DEOEC-RKEB/IKEB 5306-9/2011, 3515-2011, 3885/2012/EKU (60/PI/2012), 3880/2012/EKU (59/PI/2012)]. Each patient was informed of the nature of the study and gave informed consent in writing.
Variables were tested for normality using Shapiro Wilk’s W test. Continuous variables were summarized as medians [interquartile range (IQR, lowest 25%-highest 25%)] and were compared with the Mann-Whitney U test. Categorical variables were compared with χ2 test or Fisher’s exact test, as appropriate. We used Kaplan-Meier analysis to determine association between serological antibodies and adverse disease outcome (OLTx and/or liver-related mortality). Differences in observed survival were assessed by the log-rank test. The association of categorical clinical variables or serological antibodies with adverse disease outcome during the follow-up was evaluated by univariate Cox-regression analysis. Multivariate analyses were performed to adjust for the Mayo risk score or presence of cirrhosis with forced entry method. Associations are given as hazard ratio [HR] with 95%CI. For statistical analysis and graphical presentation, the SPSS v.22.0 (SPSS, Chicago, IL, United States) and GraphPad Prism 6 (San Diego, CA, United States) programs were used. A two-sided probability value of < 0.05 was considered to be statistically significant.
Frequencies of IgA and IgG isotype AAA and AGA in PSC and control groups comprising healthy blood-donors and patients with UC but without PSC are summarized in Table 2. A total of 40.3% (27/67) and 22.4% (15/67) of PSC patients were positive for IgA or IgG isotype AAA and AGA, respectively. Antibody prevalence was significantly higher compared to either that in patients with UC [14.7% (25/170), P < 0.001 for AAA and 11.6% (20/172), P = 0.042 for AGA] or in healthy controls [6.2% (7/113), P < 0.001 for AAA and 7.2% (11/153), P = 0.003 for AGA]. AGA positivity was mainly IgG isotype (9% vs 20.9%), while AAA was of both IgA and IgG isotypes (28.4% vs 25.4%) and there was no significant overlap between IgA and IgG subtypes of the given antibody. Likewise, no significant overlap was observed between AAA and AGA. Only 10.4% (7/67) PSC patients positive for either type of these antibodies had both AAA (IgA or IgG) and AGA (IgA or IgG) antibodies.
| PSC (n = 67) | UC (n = 172) | P value1 | HC (n = 153) | P value1 | |
| AAA | |||||
| IgA | 19 (28.4) | 19 (11.2) | 0.003 | 5 (4.4) | < 0.001 |
| IgG | 17 (25.4) | 7 (4.1) | < 0.001 | 3 (2.7) | < 0.001 |
| IgA or IgG | 27 (40.3) | 25 (14.7) | < 0.001 | 7 (6.2) | < 0.001 |
| IgA and IgG | 9 (13.4) | 1 (0.6) | < 0.001 | 1 (0.9) | 0.001 |
| AGA | |||||
| IgA | 6 (9.0) | 6 (3.5) | 0.101 | 4 (2.6) | 0.071 |
| IgG | 14 (20.9) | 15 (8.7) | 0.014 | 9 (5.9) | 0.002 |
| IgA or IgG | 15 (22.4) | 20 (11.6) | 0.042 | 11 (7.2) | 0.003 |
| IgA and IgG | 5 (7.5) | 1 (0.6) | 0.007 | 2 (1.3) | 0.029 |
We analyzed clinical and laboratory characteristics of PSC patients according to their antibody status and summarized these results in Table 3. AAA antibody status according to its immunoglobulin subtypes was not associated with gender, younger age at diagnosis, presence of cirrhosis, or concomitant IBD. However, values of several biochemical laboratory parameters and the Mayo risk score that indicate more severe disease were significantly higher in the presence of IgA isotype of AAA. In case of IgG isotype of AAA, only a single association was reported, namely the median level of ALP was significantly higher in antibody positive cases compared to antibody negative ones (715 vs 493 U/L, P = 0.048). Different AGA isotypes, however, were not associated with either the clinical or the laboratory characteristics of more severe PSC phenotype.
| Anti-F-actin IgA | Anti-F-actin IgG | Anti-gliadin IgA | Anti-gliadin IgG | |||||||||
| Negative (n = 48) | Positive (n = 19) | P value | Negative (n = 50) | Positive (n = 17) | P value | Negative (n = 61) | Positive (n = 6) | P value | Negative (n = 53) | Positive (n = 14) | P value | |
| Male gender, n (%) | 35 (72.9) | 13 (68.4) | 0.713 | 38 (76.0) | 10 (58.8) | 0.175 | 44 (72.1) | 4 (66.7) | 0.777 | 37 (69.8) | 11 (78.6) | 0.518 |
| Presence of cirrhosis, n (%) | 8 (16.7) | 5 (26.3) | 0.368 | 9 (18.0) | 4 (23.5) | 0.618 | 11 (18) | 2 (33.3) | 0.366 | 10 (18.9) | 3 (21.4) | 0.829 |
| Presence of IBD, n (%) | 36 (75) | 15 (78.9) | 0.733 | 40 (80.0) | 11 (64.7) | 0.201 | 47 (77) | 4 (66.7) | 0.569 | 40 (75.5) | 11 (78.6) | 0.809 |
| Median, IQR | ||||||||||||
| Age at diagnosis (yr) | 23 (17-33) | 26 (18-37) | 0.713 | 25 (17-37) | 20 (11-31) | 0.220 | 23 (17-35) | 23 (18-29) | 0.925 | 23 (17-37) | 22 (17-29) | 0.502 |
| Disease duration (yr) | 6 (3-8) | 7 (2-12) | 0.240 | 6 (4-10) | 6 (1-9) | 0.219 | 6 (3-9) | 9 (1-12) | 0.760 | 6 (3-8) | 6 (2-17) | 0.506 |
| Albumin (g/L) | 44 (42-46) | 39 (38-47) | 0.020 | 44 (40-46) | 43 (39-47) | 0.442 | 44 (40-47) | 39 (39-42) | 0.210 | 44 (40-46) | 43 (39-47) | 0.726 |
| Bilirubin (μmol/L) | 15 (11-20) | 14 (11-37) | 0.704 | 15 (11-20) | 13 (10-22) | 0.705 | 14 (10-22) | 17 (16-21) | 0.216 | 15 (11-23) | 17 (12-20) | 0.813 |
| AST (U/L) | 35 (25-56) | 55 (37-94) | 0.006 | 36 (27-56) | 44 (34-85) | 0.182 | 39 (28-61) | 41 (34-48) | 0.861 | 42 (28-61) | 35 (25-55) | 0.524 |
| ALT (U/L) | 46 (21-92) | 65 (45-165) | 0.030 | 50 (25-97) | 55 (32-165) | 0.532 | 52 (25-100) | 44 (37-52) | 0.294 | 54 (32-111) | 42 (24-65) | 0.188 |
| GGT (U/L) | 142 (45-269) | 193 (112-478) | 0.041 | 153 (63-305) | 153 (60-420) | 0.748 | 154 (60-310) | 140 (93-305) | 0.943 | 160 (54-310) | 126 (88-305) | 0.895 |
| ALP (U/L) | 406 (253-643) | 1198 (595-1766) | < 0.001 | 469 (268-734) | 715 (507-1496) | 0.040 | 524 (312-784) | 1204 (307-1299) | 0.336 | 507 (326-746) | 652 (268-1204) | 0.394 |
| PLT (G/L) | 238 (181-292) | 315 (162-494) | 0.212 | 235 (175-274) | 320 (162-466) | 0.066 | 248 (165-316) | 274 (232-494) | 0.292 | 253 (165-316) | 241 (187-356) | 0.751 |
| Mayo risk score | -0.834 (-1.378 to -0.131) | 0.021 (-0.554-1.248) | 0.016 | -0.62 (-1.142-0.102) | -0.226 (-1.578-0.292) | 0.623 | -0.595 (-1.194-0.102) | 0.021 (-0.843-1.327) | 0.429 | -0.579 (-1.102-0.174) | -0.651 (-1.194-0.021) | 0.762 |
The presence of AAA IgG was higher in patients with overlapping autoimmune hepatitis [55.6% (5/9) vs 20.7% (12/58), P = 0.040] compared to patients without. In contrast, the frequency of AAA IgA was not different between the two groups [33.3% (3/9) vs 27.6% (16/58), P = 0.706].
Development of colon cancer occurred in two, while biliary tract cancer in one patient during the follow-up period. Nine patients had at least one episode of cholangitis. Seven patients underwent OLTx. Five patients died due to liver-related complications. Composite end-point (OLTx and/or liver-related death) occurred in a total of 9 patients. One patient died due to acute myocardial infarction, this case was censored at time of death.
We analyzed the association of clinical variables and the different isotypes of AAA and AGA.
In Kaplan-Meier analysis, the median time to OLTx and/or liver-related death was 578 d (IQR: 212-1112). Presence of cirrhosis and increased Mayo risk score (pLogRank = 0.040 and < 0.001, respectively), but not gender (P = 0.547), age at onset (P = 0.845), disease location (P = 0.548) or concomitant IBD (P = 0.762) were significantly associated with faster disease progression.
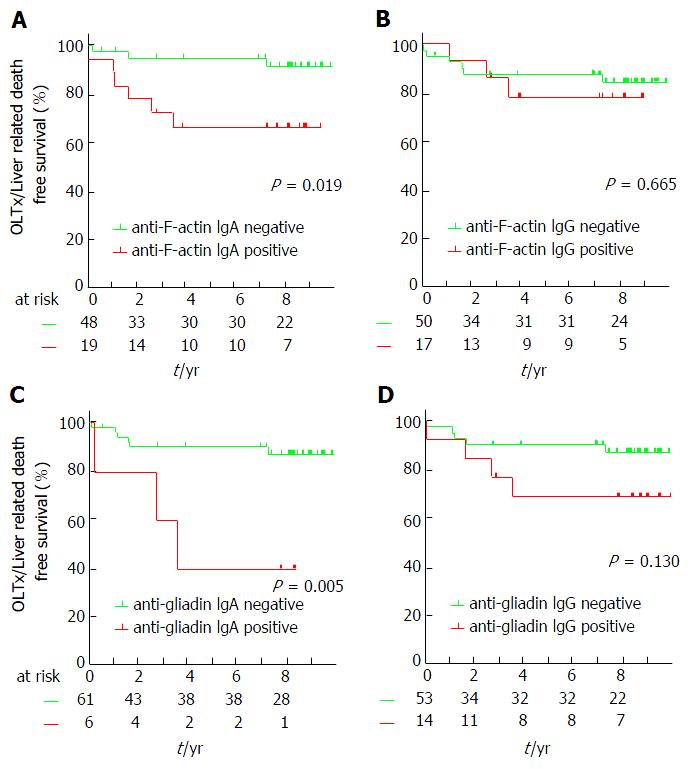
Positivity for IgA isotype AAA and AGA (pLogRank = 0.019 and 0.005, respectively), but not for IgG isotypes of these antibodies (pLogRank = 0.665 and 0.130, respectively) predicted OLTx and/or liver-related death, see Figure 1. Accordingly, univariate Cox regression analysis revealed AAA IgA and AGA IgA-positivity as predictors of poor disease outcome [HR = 4.54 (1.14-18.18), P = 0.032 and 5.83 (1.45-23.41), P = 0.013]. Thereafter, we used Cox regression analysis to adjust for important clinical variables that influence disease progression. Both IgA isotype AAA and AGA remained significant predictors of disease progression after adjusting for the presence of cirrhosis [HR = 5.15 (1.27-20.86), P = 0.022 and 5.07 (1.25-20.54), P = 0.023, respectively]. Similar results, but with weakened statistical significance was observed in the case of AAA IgA-positivity [HR = 4.24 (0.99-18.21), P = 0.052] after adjusting for the Mayo risk score, however the significant association was lost in case of AGA IgA-positivity [3.67 (0.88-15.30), P = 0.074].
As a next step, we analyzed various serological markers of bacterial translocation and enterocyte damage. Patients with AAA IgA-positivity had significantly higher EndoCab IgA titers [median (IQR): 123 (93-215) U vs 58 (40-92) U, P < 0.001] and higher frequency of anti-OMP Plus IgA antibody (36.8% vs 10.6%, P = 0.012) and also significantly higher level of the enterocyte damage marker, I-FABP [median (IQR): 365 (203-1079) pg/mL vs 166 (90-365) pg/mL, P = 0.011). However, LBP was not different between AAA IgA positive and negative patients. No other statistically significant association was found in case of AAA IgG or any isotypes of AGA (summarized in Table 4).
| Anti-F-actin IgA | Anti-F-actin IgG | Anti-gliadin IgA | Anti-gliadin IgG | |||||||||
| Negative (n = 48) | Positive (n = 19) | P value | Negative (n = 50) | Positive (n = 17) | P value | Negative (n = 61) | Positive (n = 6) | P value | Negative (n = 53) | Positive (n = 14) | P value | |
| Median, IQR | ||||||||||||
| LBP (μg/L) | 7132 (5150-9806) | 7374 (4348-12100) | 0.931 | 7377 (5795-9880) | 6847 (3828-13300) | 0.852 | 6959 (5044-9806) | 11450 (5836-15200) | 0.239 | 6959 (4951-9806) | 8090 (6077-12700) | 0.274 |
| EndoCab IgA (U) | 58 (40-92) | 123 (93-215) | < 0.001 | 68 (49-97) | 113 (52-150) | 0.195 | 70 (43-106) | 134 (66-241) | 0.079 | 70 (43-112) | 94 (52-150) | 0.301 |
| OMP Plus IgA, n (%) | 5 (10.60) | 7 (36.80) | 0.012 | 11 (22) | 1 (6.30) | 0.155 | 12 (20) | 0 (0) | 0.226 | 11 (21.20) | 1 (7.10) | 0.228 |
| I-FABP (pg/mL) | 166 (90-365) | 365 (203-1079) | 0.011 | 199 (143-513) | 300 (90-365) | 0.794 | 216 (99-443) | 342 (143-942) | 0.642 | 195 (99-379) | 342 (179-1020) | 0.092 |
The importance of gut-liver interaction in the pathogenesis of PSC has been certified by a variety of clinical and experimental evidence as discussed previously[2]. In the present study, therefore we investigated the clinical importance of different serological biomarkers related to gut barrier function in the prediction of progressive disease course in a cohort of PSC patients. To our knowledge, this is the first study examining disease outcomes in PSC according to these serological markers[19].
The cytoskeleton protein, F-actin was identified as a specific target of smooth muscle cell antibodies (SMA) in autoimmune hepatitis[20] and for over a decade IgG isotype AAA testing - either by immunofluorescence technique or ELISA - has been incorporated into the diagnostic procedure of the disease[21]. Increased occurrence of this antibody was reported in other autoimmune diseases (e.g., celiac disease or connective tissue disease)[22]. To our knowledge no previous study assessed, however, the prevalence and isotype characteristics of AAAs in PSC.
In the present study, we demonstrated - for the first time - that enhanced AAA formation is a feature of PSC regardless of overlapping AIH. A quarter of our PSC patients showed positivity for AAA that was significantly higher compared to either patients with UC or healthy controls. Contrary to routine laboratory practice, AAA was identified by anti-IgA secondary antibody in addition to anti-IgG one. This approach revealed isotype dependent association of AAA with clinical characteristics of the disease. The presence of IgA, but not IgG type AAA indicated more severe disease at baseline based on Mayo risk score and different biochemical parameters. Concordantly, previous studies in celiac disease reported that the presence of IgA isotype AAA were strongly associated with the degree of active tissue damage of the intestinal mucosa. At the same time, AAA IgA-positivity disappeared parallel to mucosal healing after gluten free diet was introduced. Of note, cases with persistent intestinal mucosa damage despite gluten-free diet, remained positive for AAA IgA antibody[23].
As a further novel finding of our study, the presence of AAA IgA-positivity predicted faster disease progression during follow-up, even after adjusting for the presence of cirrhosis or the Mayo risk score. We considered both liver-related death and OLTx as equal endpoints, since they represent the development of end-stage liver disease as the result of the progressive fibrosis.
The mechanism how the breakdown of tolerance towards F-actin is associated with the development of enhanced fibrosis and thus disease progression in the liver remaines to be elucidated. Interestingly, in our study patients with positivity for AAA IgA had an enhanced mucosal immune response to microbial antigens, like endotoxin (EndoCab IgA) or bacterial proteins (anti-OMP Plus IgA). This immune response seems to be restricted to the intestinal mucosal compartment without leading to systemic reaction since serum LBP concentration, the serologic hallmark of systemic LPS exposure were similar in AAA IgA positive and negative cases. This result is in agreement with the findings of previous studies. Namely, portal venous bacteraemia is not frequent in PSC. However, exposure of endotoxins to biliary epithelial cells leads to disruption of enterocytes’ and cholangiocytes’ tight junctions through TLR4 mediated signaling that is an important step in the pathogenesis in animal models of PSC[3,24]. Our observation that serum level of I-FABP was significantly higher in the group of patients with AAA IgA-positivity compared to those with AAA IgA-negativity corresponds to these above-mentioned literature findings. I-FABP is considered an accurate marker of enterocyte damage, since it is specifically produced by enterocytes and is released to systemic circulation in case of cellular injury during inflammatory processes.
Autoantibodies are generally not pathogenic in PSC, therefore it may be unlikely that the severity of biliary injury is driven by humoral factors. Production of AAA may reflect, however an enhanced (immune) reactivity of lymphocytes towards surrounding tissue and/or cellular debris. Of note, AAA is not organ-specific since they may be present in a wide range of immune-mediated diseases other than PSC, as mentioned previously. IgG isotype AAA was associated with disease activity and poor survival in autoimmune hepatitis[25,26]. In the study of Czaja et al[26]patients seropositive for AAA were more commonly HLA-DR3 positive, while seronegative patients had higher frequency of HLA-DR4 positivity than healthy subjects. In the same study AAA were also associated with HLA-B8 positivity. Interestingly, in PSC Wiencke et al[27] also demonstrated that HLA-DR3 and B8 are associated with progressive disease course. Conversely, patients with HLA-DR4 do not experience an accelerated disease progression, they also have a decreased risk for disease recurrence after liver transplantation[28,29]. Based on these previous findings we speculate that the formation of AAA IgA in PSC might reflect a phenotype with distinct immunological function and genetic susceptibility for a more severe inflammatory process, similarly as in autoimmune hepatitis. A recent report on the association between autoantibodies, like atypical P-ANCA and HLA status in PSC might further support this hypothesis[30]. Hypothetical sero-genotype linkage between AAA IgA-positivity and more aggressive HLA genotype warrants further exploration.
In the present study, occurrence of AGA was significantly higher in patients with PSC compared to either that in patients with UC or healthy subjects. Frequency of AGA IgG/IgA corresponds to the findings of Sjöberg et al[31] (22.4% vs 24%). Distinctly only IgG but not IgA isotype were more frequent in our study. Higher frequency of AGA is observed in various disorders, such as celiac disease, neurodegenerative diseases, systemic lupus erythematosus and autoimmune liver diseases[32-34]. Investigators have attributed the formation of these antibodies to an increased uptake of peptides from the gut lumen to the intestinal mucosa and presence of AGA is considered as a marker of a non-specific immune reaction towards a dietary peptide. Furthermore, Reiberger et al[14] reported a surprisingly high frequency (about 60%) of AGA in patients with liver cirrhosis, mainly of alcoholic origin. Patients with AGA had higher portal venous pressure and increased intestinal permeability assessed by the sucrose-lactulose-mannitol test. Nevertheless, a Swedish study including 22 patients with PSC failed to detect an altered intestinal permeability in PSC[35]. Intestinal permeability in PSC might rather be considered as an immunological barrier dysfunction[8,36]. We found an association between IgA type AGA and accelerated disease progression in our cohort, however due to the low number of positive cases, this finding should be interpreted with caution. The increased chance of a false positive finding as a result of the small number of positive cases is a limitation to be acknowledged. We speculate that the finding of an earlier study from a French research group might serve as a possible explanation. They showed that AGA IgA facilitated an increased transport of gliadin peptides from the intestinal lumen to the gut mucosa via CD71. In the subepithelial space these undigested toxic peptides are able to perpetuate intestinal inflammation[37]. Of note, the design of our study was mainly explorative and did not include experimental methods that could give an exact explanation for the increased risk of disease progression in the presence of specific antibodies.
To conclude, we reported that antibodies against F-actin are frequent in patients with PSC. Presence of IgA isotype of this antibody was associated with an enhanced mucosal immune response to various microbial antigens and also with the presence of enterocyte damage. Furthermore, AAA IgA identified a subgroup of patients with an increased risk of accelerated disease progression. These results serve as additional proof for the importance of the gut-liver interaction in PSC. If they confirmed in large-scale cohorts, IgA isotype of AAA could be a candidate biomarker for serological risk stratification of patients with PSC.
Clinical manifestation and progression of primary sclerosing cholangitis are heterogeneous. A large body of clinical evidence has certified the importance of the gut-liver interaction in primary sclerosing cholangitis (PSC): (1) inflammatory bowel diseases (IBD) is associated to PSC in up to 80% of the patients; (2) high peritransplant IBD activity increases the risk, while colectomy before liver transplantation decreases the risk of disease recurrence after transplantation; and (3) patients with PSC display an exaggerated immune response to endotoxins with a lack of tolerance to repeated exposure. To date, no widely used biomarkers are able to predict rapid progression of PSC.
A cytoplasmic protein of enterocytes, intestinal fatty acid-binding protein (I-FABP) is as marker of enterocyte damage. Anti-F-actin IgA antibodies were showed to serve as the markers of the structural intestinal mucosal damage. Presence of anti-gliadin IgA antibodies was associated with increased intestinal permeability in patients with cirrhosis and significant portal hypertension. In the present study, they tested these different novel serological biomarkers related to gut barrier function in the prediction of progressive disease course in a cohort of PSC patients.
In the present study, they demonstrated - for the first time - that enhanced AAA IgA formation is a feature of PSC. A quarter of their PSC patients showed positivity for AAA IgA. Immune response to microbial antigens was more frequent in patients with AAA IgA. The presence of AAA IgA predicted faster disease progression during follow-up, even when adjusting for the presence of cirrhosis or the Mayo risk score.
AAA IgA is a candidate biomarker for stratifying patients with PSC in terms of risk of disease progression.
AAA IgA is an autoantibody of immunoglobulin A isotype directed against filamentous actin.
This is an original and interesting manuscript. There are very few data on this topic.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bonaz B, Popp C S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375:1161-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 2. | Eksteen B. The Gut-Liver Axis in Primary Sclerosing Cholangitis. Clin Liver Dis. 2016;20:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 271] [Article Influence: 24.6] [Reference Citation Analysis (36)] |
| 4. | Joshi D, Bjarnason I, Belgaumkar A, O’Grady J, Suddle A, Heneghan MA, Aluvihare V, Rela M, Heaton N, Agarwal K. The impact of inflammatory bowel disease post-liver transplantation for primary sclerosing cholangitis. Liver Int. 2013;33:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, Neuberger J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Lichtman SN, Keku J, Schwab JH, Sartor RB. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513-519. [PubMed] [Cited in This Article: ] |
| 7. | Yamada S, Ishii M, Liang LS, Yamamoto T, Toyota T. Small duct cholangitis induced by N-formyl L-methionine L-leucine L-tyrosine in rats. J Gastroenterol. 1994;29:631-636. [PubMed] [Cited in This Article: ] |
| 8. | Mueller T, Beutler C, Picó AH, Shibolet O, Pratt DS, Pascher A, Neuhaus P, Wiedenmann B, Berg T, Podolsky DK. Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver Int. 2011;31:1574-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Fickert P, Fuchsbichler A, Marschall HU, Wagner M, Zollner G, Krause R, Zatloukal K, Jaeschke H, Denk H, Trauner M. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol. 2006;168:410-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261-274. [PubMed] [Cited in This Article: ] |
| 12. | Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care. 2016;22:152-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Schirru E, Danjou F, Cicotto L, Rossino R, Macis MD, Lampis R, Jores RD, Congia M. Anti-actin IgA antibodies identify celiac disease patients with a Marsh 3 intestinal damage among subjects with moderate anti-TG2 levels. Biomed Res Int. 2013;2013:630463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-radosavljevic M, Vogelsang H. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 15. | Martins EB, Chapman RW. Sclerosing cholangitis. Curr Opin Gastroenterol. 2001;17:458-462. [PubMed] [Cited in This Article: ] |
| 16. | Kim WR, Therneau TM, Wiesner RH, Poterucha JJ, Benson JT, Malinchoc M, LaRusso NF, Lindor KD, Dickson ER. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Papp M, Sipeki N, Tornai T, Altorjay I, Norman GL, Shums Z, Roggenbuck D, Fechner K, Stöcker W, Antal-Szalmas P. Rediscovery of the Anti-Pancreatic Antibodies and Evaluation of their Prognostic Value in a Prospective Clinical Cohort of Crohn’s Patients: The Importance of Specific Target Antigens [GP2 and CUZD1]. J Crohns Colitis. 2015;9:659-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Papp M, Sipeki N, Vitalis Z, Tornai T, Altorjay I, Tornai I, Udvardy M, Fechner K, Jacobsen S, Teegen B. High prevalence of IgA class anti-neutrophil cytoplasmic antibodies (ANCA) is associated with increased risk of bacterial infection in patients with cirrhosis. J Hepatol. 2013;59:457-466. [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Infantino M, Meacci F, Grossi V, Macchia D, Manfredi M. Anti-gliadin antibodies in non-celiac gluten sensitivity. Minerva Gastroenterol Dietol. 2017;63:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Gabbiani G, Ryan GB, Lamelin JP, Vassalli P, Majno G, Bouvier CA, Cruchaud A, Lüscher EF. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to „nonmuscular” cells. Am J Pathol. 1973;72:473-488. [PubMed] [Cited in This Article: ] |
| 21. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1205] [Cited by in F6Publishing: 1178] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 22. | Chretien-Leprince P, Ballot E, Andre C, Olsson NO, Fabien N, Escande A, Oksman F, Dubuquoi S, Jego S, Goetz J. Diagnostic value of anti-F-actin antibodies in a French multicenter study. Ann N Y Acad Sci. 2005;1050:266-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Clemente MG, Musu MP, Troncone R, Volta U, Congia M, Ciacci C, Neri E, Not T, Maggiore G, Strisciuglio P. Enterocyte actin autoantibody detection: a new diagnostic tool in celiac disease diagnosis: results of a multicenter study. Am J Gastroenterol. 2004;99:1551-1556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 435] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 25. | Couto CA, Bittencourt PL, Porta G, Abrantes-Lemos CP, Carrilho FJ, Guardia BD, Cançado EL. Antismooth muscle and antiactin antibodies are indirect markers of histological and biochemical activity of autoimmune hepatitis. Hepatology. 2014;59:592-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Czaja AJ, Cassani F, Cataleta M, Valentini P, Bianchi FB. Frequency and significance of antibodies to actin in type 1 autoimmune hepatitis. Hepatology. 1996;24:1068-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 123] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Wiencke K, Spurkland A, Schrumpf E, Boberg KM. Primary sclerosing cholangitis is associated to an extended B8-DR3 haplotype including particular MICA and MICB alleles. Hepatology. 2001;34:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Bowlus CL, Li CS, Karlsen TH, Lie BA, Selmi C. Primary sclerosing cholangitis in genetically diverse populations listed for liver transplantation: unique clinical and human leukocyte antigen associations. Liver Transpl. 2010;16:1324-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Boberg KM, Spurkland A, Rocca G, Egeland T, Saarinen S, Mitchell S, Broomé U, Chapman R, Olerup O, Pares A. The HLA-DR3,DQ2 heterozygous genotype is associated with an accelerated progression of primary sclerosing cholangitis. Scand J Gastroenterol. 2001;36:886-890. [PubMed] [Cited in This Article: ] |
| 30. | Hov JR, Boberg KM, Taraldsrud E, Vesterhus M, Boyadzhieva M, Solberg IC, Schrumpf E, Vatn MH, Lie BA, Molberg Ø, Karlsen TH. Antineutrophil antibodies define clinical and genetic subgroups in primary sclerosing cholangitis. Liver Int. 2017;37:458-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Sjöberg K, Lindgren S, Eriksson S. Frequent occurrence of non-specific gliadin antibodies in chronic liver disease. Endomysial but not gliadin antibodies predict coeliac disease in patients with chronic liver disease. Scand J Gastroenterol. 1997;32:1162-1167. [PubMed] [Cited in This Article: ] |
| 32. | Chatzicostas C, Roussomoustakaki M, Drygiannakis D, Niniraki M, Tzardi M, Koulentaki M, Dimoulios P, Mouzas I, Kouroumalis E. Primary biliary cirrhosis and autoimmune cholangitis are not associated with coeliac disease in Crete. BMC Gastroenterol. 2002;2:5. [PubMed] [Cited in This Article: ] |
| 33. | Reichelt KL, Jensen D. IgA antibodies against gliadin and gluten in multiple sclerosis. Acta Neurol Scand. 2004;110:239-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Rensch MJ, Szyjkowski R, Shaffer RT, Fink S, Kopecky C, Grissmer L, Enzenhauer R, Kadakia S. The prevalence of celiac disease autoantibodies in patients with systemic lupus erythematosus. Am J Gastroenterol. 2001;96:1113-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Björnsson E, Cederborg A, Akvist A, Simren M, Stotzer PO, Bjarnason I. Intestinal permeability and bacterial growth of the small bowel in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2005;40:1090-1094. [PubMed] [Cited in This Article: ] |
| 36. | Terjung B, Söhne J, Lechtenberg B, Gottwein J, Muennich M, Herzog V, Mähler M, Sauerbruch T, Spengler U. p-ANCAs in autoimmune liver disorders recognise human beta-tubulin isotype 5 and cross-react with microbial protein FtsZ. Gut. 2010;59:808-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Ménard S, Candalh C, Ben-Khalifa K, Dugave C, Tamouza H, van Niel G. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143 LP-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |