Copyright
©The Author(s) 2017.
World J Gastroenterol. Jul 21, 2017; 23(27): 4910-4919
Published online Jul 21, 2017. doi: 10.3748/wjg.v23.i27.4910
Published online Jul 21, 2017. doi: 10.3748/wjg.v23.i27.4910
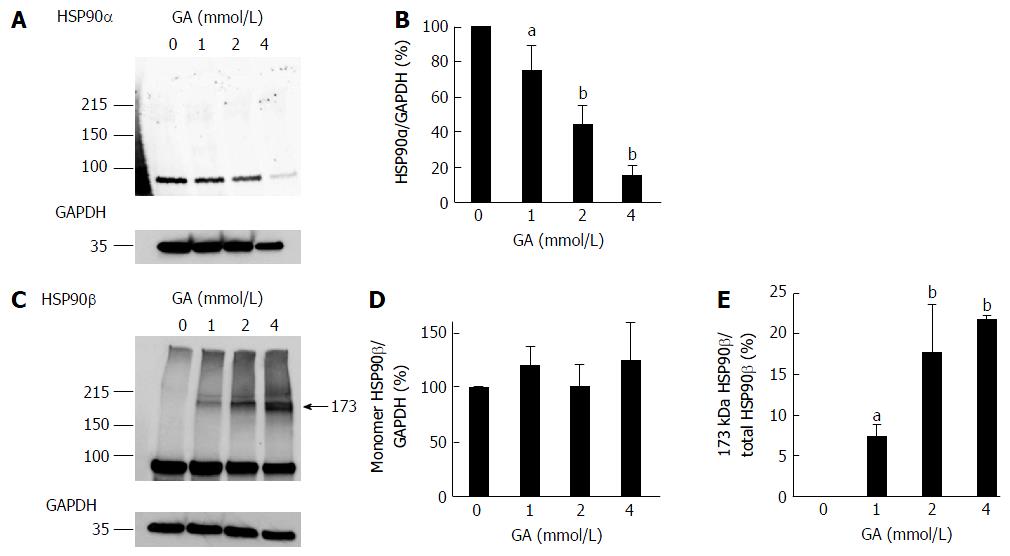
Figure 2 Western blotting analysis of HSP90α and HSP90β.
PANC-1 cell lysates (15 μg of proteins/lane) were loaded on a 40-150 g/L polyacrylamide gradient gel. A: Proteins on the polyvinylidene difluoride (PVDF) membrane were probed with anti-HSP90α and anti-GAPDH antibodies; B: Expression levels of HSP90α were normalized with GAPDH; C: Proteins on the PVDF membrane were probed with anti-HSP90β and anti-GAPDH antibodies. A band of 173 kDa HSP90β only appeared in PANC-1 cells treated with GA; D: Expression levels of the monomer HSP90β were normalized with GAPDH; E: The 173 kDa HSP90β/total HSP90β ratio. A and C: Western blotting was performed for three independent experiments. GAPDH was used as the loading control; B, D and E: Data are shown as mean ± SD (n = 3). P values were based on Dunnett’s test. aP < 0.05, bP < 0.01 vs control. GA: Glyceraldehyde.
- Citation: Takata T, Ueda T, Sakasai-Sakai A, Takeuchi M. Generation of glyceraldehyde-derived advanced glycation end-products in pancreatic cancer cells and the potential of tumor promotion. World J Gastroenterol 2017; 23(27): 4910-4919
- URL: https://www.wjgnet.com/1007-9327/full/v23/i27/4910.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i27.4910