Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4140
Peer-review started: February 8, 2017
First decision: March 16, 2017
Revised: April 3, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: June 21, 2017
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been recently demonstrated as a method to induce rapid and extensive hypertrophy within a short time and has been employed for a variety of primary and metastatic liver tumors. However, controversies remain due to its high morbidity and mortality. To enable safer surgery, liver surgeons have searched for better technical modifications, such as partial ALPPS, mini-ALPPS, minimally invasive ALPPS, and Terminal branches portal vein Embolization Liver Partition for Planned hepatectomy (TELPP). It seems that TELPP is very promising, because it has the main advantage of ALPPS - the rapid increase of future liver remnant volume, but the morbidity and mortality are much lower because only one surgical operation is required.
Core tip: Many technical modifications have been proposed for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) due to its high morbidity and mortality. We described a new one, named Terminal branches portal vein Embolization Liver Partition for Planned hepatectomy, which uses a different method to interrupt the communicating portal vein branches, not by manipulation of the liver parenchyma but by the implementation of the embolization of terminal portal vein branches between both sides of the liver. It has the main advantage of ALPPS - the rapid increase of future liver remnant volume, but the morbidity and mortality are much lower.
- Citation: Peng SY, Wang XA, Huang CY, Zhang YY, Li JT, Hong DF, Cai XJ. Evolution of associating liver partition and portal vein ligation for staged hepatectomy: Simpler, safer and equally effective methods. World J Gastroenterol 2017; 23(23): 4140-4145
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4140.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4140
Associating liver partition and portal vein ligation (PVL) for staged hepatectomy (ALPPS) is a novel two-stage strategy for oncological liver surgery that was developed to induce future liver remnant (FLR) hypertrophy in patients with previously considered nonresectable liver tumors[1,2]. This great new approach was invented by chance by Schlitt in 2007 for a patient with hilar cholangiocarcinoma who was about to undergo right trisegmentectomy. Intraoperatively, it was found that the FLR would be insufficient. Then, he split the liver parenchyma along the falciform ligament to facilitate the left hepaticojejunostomy for palliation together with PVL to induce hypertrophy of the left lateral lobe. Finally, he performed the second stage operation to resect the diseased liver because the computed tomography (CT) scan showed enormous hypertrophy of the FLR on postoperative day 8. The patient recovered from the operation[2].
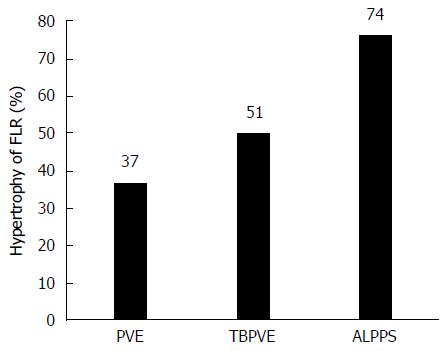
This technique was first reported by Baumgart et al[3] on a poster. In 2012, Schnitzbauer et al[1] published their experience with 25 cases performed in 5 German centers with a median FLR hypertrophy of 74% after a median time interval of 9 d. This value was markedly higher than that for portal vein occlusion (PVE or PVL), which increases the FLR between 10% to 46% within 2 to 8 wk[4-6]. The article attracted significant interest from liver surgeons worldwide, and de Santibañes and Clavien[2] proposed the term “ALPPS” for this novel approach. In that article, the authors also revealed a hospital lethality of 12% and a 24% rate of biliary leakage requiring radiologic or endoscopic intervention. Then, an international registry was initiated to collect information from multiple centers worldwide from 2012 to monitor the feasibility and safety of ALPPS. The first analysis of 202 patients by Schadde et al[7] in January 2014 reported a perioperative 90-d mortality of 9% and an impressive hypertrophy of 80% within a median of 7 d. The high mortality rate has elicited an intense discussion and debate about the safety of ALPPS[8,9].
Even though ALPPS is significantly characterized by increasing the insufficient remnant liver volume within a shorter interval for two-stage resection, much controversy has surrounded it due to its safety; for example, the reported remarkable morbidity was 16%-64% and the mortality rate was 12%-23%[10-13]. Barroso considered ALPPS as the last option, or the ultimate possibility to cure some patients; he thought that it was not ethical to propose this kind of operation to a patient without first proposing a PVE[14]. Thus, to reduce the perioperative mortality and morbidity rates, to achieve a long-term disease-free survival and to enable safer surgery, liver surgeons have searched for better technical modifications.
Based on their experimental model, Petrowsky et al[15] developed a technical modification, named p-ALPPS (partial ALPPS), to switch from full liver partition to partial transection (50%-80% of the transection surface). They compared 18 patients who underwent full transection with 6 patients who underwent 50% to 80% partition, and the results displayed a comparable degree of liver hypertrophy on postoperative day 11 with fewer severe complications (Dindo-Clavien grade ≥ 3b) and zero in-hospital mortality. Alvarez et al[16] confirmed the value of p-ALPPS by a prospectively multivariate analysis that included 21 patients who underwent partial partition. In addition, they defined partial partition as the level of the middle hepatic vein, whereas total partition as the vena cava. However, a partial partition during the first stage will challenge the second stage, as it requires longer liver parenchymal transection.
Hernandez-Alejandro et al[17] have cautioned that extensive dissection of the hepatoduodenal ligament increases the likelihood of segment 4 ischemia and potentially increases the risk of bile leakage and resulting septic complications. Consistent with this, Tanaka et al[18] considered that sepsis originating from the ischemic area produced by parenchymal division increases mortality, accounting for one-third of postoperative deaths. They described a modified ALPPS procedure that preserves the portal pedicles during parenchymal division to avoid producing an ischemic area. Five patients received this modification without mortality. Mean hypertrophy of the FLR was 1.638 ± 0.384 a week after the first stage procedure.
de Santibañes et al[19] proposed mini-ALPPS, which combined partial transection and intraoperative PVE without hilum dissection or liver mobilization during the first stage. They applied this technique in four patients with a result of 62.6% (range, 49%-79%) FLR hypertrophy in a median of 11 d (range, 6-15 d), and no one developed liver failure or major complications.
The prominent advantage of ALPPS is the rapid and extensive hypertrophy of FLR within a short time period; however, the morbidity rate and the in-hospital mortality rate are incredibly high, which constitutes a major concern. Of note, septic complications and bile leakage were observed in 20%-25% of patients. Obviously, bile leakage stems from the two raw surfaces of the split liver that are left behind in the abdominal cavity after the first stage operation. The bile leakage may result in septic complications. Therefore, the avoidance of liver parenchymal division is important. According to Alvarez et al[12], the mechanisms for rapid FLR hypertrophy might be because: (1) PVL creates a redistribution of hepatotrophic factors to the FLR, and this produces the active and necessary phenomenon of FLR hypertrophy; (2) liver partition causes local surgical trauma that per se might represent an important regeneration stimulus; (3) the impairment of bilateral cross-portal circulation allows a more dramatic increase in portal flow to the FLR; and (4) unlike one-stage major hepatectomies, in which the liver remnant has to address hyper flow and portal hypertension, in this technique the diseased arterialized hemiliver allows the FLR to tolerate this hemodynamic stress, modulating the double hepatic vascular inflow.
Based on the third mechanism, liver parenchyma division can be avoided, so long as bilateral cross-portal circulation can be blocked by other methods. These methods are described below.
Using a liver tourniquet: In a 2013 case report, Robles Campos et al[20] described a modification termed ALTPS (associating liver tourniquet and portal ligation for staged hepatectomy) with a hypertrophy of 150% of the FLR. Instead of an in situ split, a tourniquet was positioned around the liver following either Cantlie’s line or to the right of the umbilical fissure for the first stage of ALPPS. This tourniquet was then tied tightly enough to occlude all collateral vessels between the two lobes, which was confirmed by intraoperative ultrasound (IOUS). This modified approach might potentially decrease morbidity by decreasing technical complexity and shortening the operative time for the first stage of the operation.
Cai et al[21] adopted the execution of round-the-liver ligation to replace the in situ splitting of the liver, which could avoid postoperative bile leakage and might simplify the operation. The FLR volume increased by 37.9% according to the CT scan performed on day 10 after the first stage operation. The replacement of liver splitting by round-the-liver ligation could avoid biliary leakage, simplify the first stage operation and finally lead to a decrease in perioperative morbidity and mortality. Both the first and second stage operations were performed laparoscopically.
Using microwave liver ablation: Another new technique to avoid liver parenchymal division was presented by Gall et al[22]. After right PVL, an inline radiofrequency ablation (RFA) probe was applied to the parenchyma instead of the in situ liver partition. The hypertrophy of the FLR was 62.3% at a mean interval of 21.8 d. In 2015, Gringeri et al[23] described laparoscopic microwave ablation and PVL for staged hepatectomy (LAPS) on the future transection plane with a satisfactory hypertrophy of FLR and an easier second step in hepatocellular cancer (HCC) 10 d later. Compared with the traditional ALPPS, this technique may offer some advantages, such as an easier second operation due to the lack of adhesions and safer liver resection along the avascular groove.
Hong et al[24] presented a novel minimally invasive approach implementing percutaneous microwave ablation liver partition and portal vein embolization (PALPP) instead of the first step of ALPPS for rapid liver regeneration. The authors applied percutaneous microwave ablation (PMA) every 3 cm along the transection plane under ultrasonographic guidance until the formation of a necrotic groove from the inferior liver to the suprahepatic veins. The PMA line was positioned on the right side of the transection plane at a power of 60 W set as a 3-min ablation cycle. PVE was performed 3 d after PMA. Fourteen days later, a well-planned right trisectionectomy was performed. Three cases of HCC and 1 case of hilar cholangiocarcinoma were performed using this approach with a hypertrophic rate of 41.2%, which was similar to the results for HCC[25]. Hong’s approach may have additional benefits. Tumor spread caused by ALPPS could be mitigated and intraoperative and postoperative bleeding along with bile leakage could be reduced as a result of microscopically coagulative necrosis.
Original PVE only requires one operation, but the proliferative speed is too slow. However, the ALPPS and all modifications require two-stage operations with high morbidity and mortality rates. Is it possible to merge the concepts of ALPPS and PVE for designing a simpler and safer technique? It would be preferred to perform a single surgical operation rather than two to achieve the same therapeutic effect.
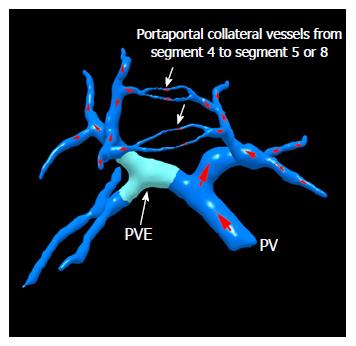
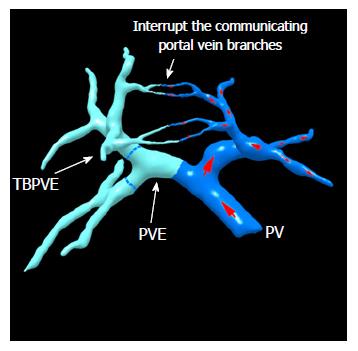
TELPP: The aforementioned special modifications of ALPPS to avoid liver parenchymal division have proven that the blockage of bilateral cross-portal circulation can promote FLR hypertrophy. Trying to search for a better way, we proposed terminal branches portal vein embolization (TBPVE) by applying an additional embolization agent on the endings of the portal vein system of S5, S8 or S4 (Figures 1 and 2).
TBPVE uses a different method to interrupt the communicating portal vein branches, not by manipulation of the liver parenchyma but rather by the implementation of the embolization of terminal portal vein branches between both sides of the liver. The mechanism of TBPVE is to separate the left and right sides of the liver by blocking communicating branches. All blood in the portal vein on one side is diverted to the other side, and consequently the remnant liver proliferates at a speed comparable to that after ALPPS. There is no need to manipulate the liver parenchyma, such as the division of liver parenchyma, placing a liver tourniquet, or executing liver ablation. Thus, only a single surgical operation is needed. In the initial report, four patients who underwent this procedure followed by right hemi-hepatectomy two weeks later did not have mortality and severe morbidity. The mean hypertrophy was 52.2% (68.4%, 33.1%, 54.2% and 53.1%, respectively), which was similar to that with ALPPS[26,27]. Currently, TBPVE has been performed for 20 cases, and the mean rate of volume increase was 51% (Figure 3, unpublished data).
Based on these preliminary practices, TBPVE can effectively increase FLR similarly to ALPPS, but much less invasively. This indicates that TBPVE is simple, safe and effective and is able to avoid some disadvantages of ALPPS. It only needs one interventional manipulation and a single surgical operation to achieve a similar therapeutic effect to ALPPS. We propose naming it “Terminal branches portal vein Embolization Liver Partition for Planned hepatectomy (TELPP)”. The efficacy and safety of this new technique is expected to be verified by a large-scale, multi-center study.
TBPVE combined with TACE: There is concern about tumor growth during the lag between PVE and surgical operations, as hepatic arterial flow might increase to promote the tumor growth. Kokudo et al[28] described an increase of the tumor Ki-67 labeling index in intrahepatic metastases in the embolized liver after PVE. The same phenomenon was also noted with ALPPS. Fukami et al[29] reported an increase in the Ki-67 labeling index from 60% at the first stage to 80% at the second stage by tumor biopsy results of the same liver lesion at both stages. TBPVE also raises the same concern, but not as strongly as PVE. This problem was solved by performing TBPVE combined with TACE. When we used this method in four cases, the average rate of FLR volume increase was 68.6%, while the tumor mass shrank.
Recently, Chao et al[30] found that lactic acidosis could effectively protect cancer cells against glucose starvation or deprivation and recruited 20 patients for a randomized trial to compare embolization alone with embolization plus bicarbonate treatment (TILA-TACE). The results showed that the tumors died more and patients survived longer if they received the bicarbonate. These data indicate that bicarbonate markedly enhances the anticancer activity of TACE. This therapy may be effective for patients with large tumors that are not amenable to surgery. Next, we would like to combine TBPVE with TILA-TACE to determine whether it could provide more benefit for patients with liver tumors previously considered nonresectable.
ALPPS is a revolutionary two-stage surgical procedure for the resection of hepatic malignancies, which has attracted the attention of many hepato-biliary surgeons around the world. Many modifications have been proposed to reduce its high morbidity and mortality rates. So far, we found that TELPP, which applies and merges the concepts of ALPPS and PVE to perform TBPVE, may be a promising procedure. TBPVE combined with TILA-TACE is even better in view of the tumor growth problem during the lag before hepatectomy. Their technical feasibility, safety and oncological outcomes need to be verified further in a larger-scale and multi-center study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen CY S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 930] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 2. | de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255:415-417. [PubMed] [Cited in This Article: ] |
| 3. | Baumgart J, Lang S, Lang H. A new method for induction of liver hyper- trophy prior to right trisectionectomy: a report of three cases. HPB (Oxford). 2011;13:1-145. [Cited in This Article: ] |
| 4. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 5. | Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009;197:686-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 16] [Reference Citation Analysis (0)] |
| 6. | Wicherts DA, de Haas RJ, Andreani P, Sotirov D, Salloum C, Castaing D, Adam R, Azoulay D. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br J Surg. 2010;97:240-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829-836; discussion 836-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 321] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 8. | Dokmak S, Belghiti J. Which limits to the “ALPPS” approach? Ann Surg. 2012;256:e6; author reply e16-e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Aloia TA, Vauthey JN. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost? Ann Surg. 2012;256:e9; author reply e16-e19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Sala S, Ardiles V, Ulla M, Alvarez F, Pekolj J, de Santibañes E. Our initial experience with ALPPS technique: encouraging results. Updates Surg. 2012;64:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Li J, Girotti P, Königsrainer I, Ladurner R, Königsrainer A, Nadalin S. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg. 2013;17:956-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Alvarez FA, Ardiles V, Sanchez Claria R, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg. 2013;17:814-821. [PubMed] [Cited in This Article: ] |
| 13. | Torres OJ, Fernandes Ede S, Oliveira CV, Lima CX, Waechter FL, Moraes-Junior JM, Linhares MM, Pinto RD, Herman P, Machado MA. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig. 2013;26:40-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Conrad C, Shivathirthan N, Camerlo A, Strauss C, Gayet B. Laparoscopic portal vein ligation with in situ liver split for failed portal vein embolization. Ann Surg. 2012;256:e14-e15; author reply e16-e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Petrowsky H, Györi G, de Oliveira M, Lesurtel M, Clavien PA. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg. 2015;261:e90-e92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Alvarez FA, Ardiles V, de Santibañes M, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg. 2015;261:723-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 17. | Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, Croome KP. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery. 2015;157:194-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Tanaka K, Kikuchi Y, Kawaguchi D, Murakami T, Hiroshima Y, Matsuo K. Modified ALPPS Procedures Avoiding Division of Portal Pedicles. Ann Surg. 2017;265:e14-e20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | de Santibañes E, Alvarez FA, Ardiles V, Pekolj J, de Santibañes M. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini-ALPPS technique. Langenbecks Arch Surg. 2016;401:557-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Robles Campos R, Parrilla Paricio P, López Conesa A, Brusadín R, López López V, Jimeno Griñó P, Fuster Quiñonero M, García López JA, de la Peña Moral J. A new surgical technique for extended right hepatectomy: tourniquet in the umbilical fissure and right portal vein occlusion (ALTPS). Clinical case. Cir Esp. 2013;91:633-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Cai X, Peng S, Duan L, Wang Y, Yu H, Li Z. Completely laparoscopic ALPPS using round-the-liver ligation to replace parenchymal transection for a patient with multiple right liver cancers complicated with liver cirrhosis. J Laparoendosc Adv Surg Tech A. 2014;24:883-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Gall TM, Sodergren MH, Frampton AE, Fan R, Spalding DR, Habib NA, Pai M, Jackson JE, Tait P, Jiao LR. Radio-frequency-assisted Liver Partition with Portal vein ligation (RALPP) for liver regeneration. Ann Surg. 2015;261:e45-e46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Gringeri E, Boetto R, D’Amico FE, Bassi D, Cillo U. Laparoscopic microwave ablation and portal vein ligation for staged hepatectomy (LAPS): a minimally invasive first-step approach. Ann Surg. 2015;261:e42-e43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Hong DF, Zhang YB, Peng SY, Huang DS. Percutaneous Microwave Ablation Liver Partition and Portal Vein Embolization for Rapid Liver Regeneration: A Minimally Invasive First Step of ALPPS for Hepatocellular Carcinoma. Ann Surg. 2016;264:e1-e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Chan AC, Poon RT, Chan C, Lo CM. Safety of ALPPS Procedure by the Anterior Approach for Hepatocellular Carcinoma. Ann Surg. 2016;263:e14-e16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Peng SY, Huang CY, Li JT, Zhang YY, He XW, Wang YF, Hong DF, Cai XJ. Terminal branches portal vein embolization for planed hepatectomy. Zhonghua Waike Zazhi. 2016;54:664-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 27. | Hasselgren K, Sandström P, Björnsson B. Role of associating liver partition and portal vein ligation for staged hepatectomy in colorectal liver metastases: a review. World J Gastroenterol. 2015;21:4491-4498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 278] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Fukami Y, Kurumiya Y, Kobayashi S. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): an analysis of tumor activity. Updates Surg. 2014;66:223-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Chao M, Wu H, Jin K, Li B, Wu J, Zhang G, Yang G, Hu X. A nonrandomized cohort and a randomized study of local control of large hepatocarcinoma by targeting intratumoral lactic acidosis. Elife. 2016;5:e15691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |