Copyright
©The Author(s) 2017.
World J Gastroenterol. May 21, 2017; 23(19): 3418-3426
Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3418
Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3418
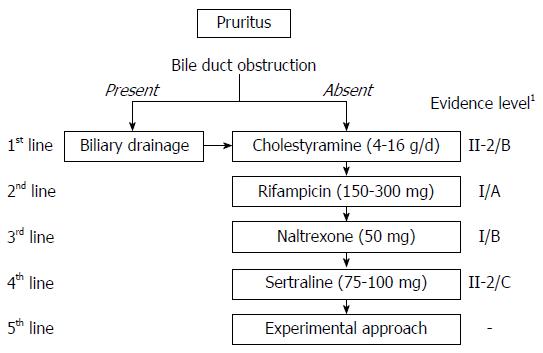
Figure 2 Therapeutic recommendations for the management of cholestatic pruritus modified from American Association for the Study of Liver Diseases guidelines.
1Evidence level involves categories of evidence and evidence grading. Categories of evidence include: I, randomized controlled trials; II-1 controlled trials without randomization; II-2, cohort and case-control analytic studies; II-3, multiple time series, dramatic uncontrolled experiments; and III, opinions of respected authorities, descriptive epidemiology. Evidence grading includes A: High quality, indicating that further research is unlikely to change confidence in the estimate of effect; B: Moderate quality, indicating that further research may have an important impact on confidence in the estimate of effect and may change that estimate; and C: Low quality, indicating that further research is very likely to have an important impact on confidence in the estimation of effect and is likely to change that estimate. Any change of estimate is uncertain.
- Citation: Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol 2017; 23(19): 3418-3426
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3418