Published online Apr 14, 2017. doi: 10.3748/wjg.v23.i14.2545
Peer-review started: December 29, 2016
First decision: February 10, 2017
Revised: February 27, 2017
Accepted: March 21, 2017
Article in press: March 21, 2017
Published online: April 14, 2017
Processing time: 107 Days and 4.3 Hours
To compare the performances of the Barcelona clinic liver cancer (BCLC) nomogram and others systems (BCLC, HKLC, CLIP, NIACE) for survival prediction in a large hepatocellular carcinoma (HCC) French cohort.
Data were collected retrospectively from 01/2007 to 12/2013 in five French centers. Newly diagnosed HCC patients were analyzed. The discriminatory ability, homogeneity ability, prognostic stratification ability Akaike information criterion (AIC) and C-index were compared among scoring systems.
The cohort included 1102 patients, mostly men, median age 68 [60-74] years with cirrhosis (81%), child-Pugh A (73%), alcohol-related (41%), HCV-related (27%). HCC were multinodular (59%) and vascular invasion was present in 41% of cases. At time of HCC diagnosis BCLC stages were A (17%), B (16%), C (60%) and D (7%). First line HCC treatment was curative in 23.5%, palliative in 59.5%, BSC in 17% of our population. Median OS was 10.8 mo [4.9-28.0]. Each system distinguished different survival prognosis groups (P < 0.0001). The nomogram had the highest discriminatory ability, the highest C-index value. NIACE score had the lowest AIC value. The nomogram distinguished sixteen different prognosis groups. By classifying unifocal large HCC into tumor burden 1, the nomogram was less powerful.
In this French cohort, the BCLC nomogram and the NIACE score provided the best prognostic information, but the NIACE could even help treatment strategies.
Core tip: Barcelona clinic liver cancer (BCLC) nomogram was compared with BCLC, HKLC systems, CLIP, and NIACE scores for survival prediction in a HCC French cohort. 1102 patients were retrospectively included, with cirrhosis (81%), child-Pugh A (73%). Hepatocellular carcinoma (HCC) were multinodular (59%) and with vascular invasion (41%). At time of HCC diagnosis, patients were mainly BCLC-C (60%). First line HCC treatment was curative (23.5%) or palliative (59.5%). Median OS was 10.8 mo [4.9-28.0]. BCLC nomogram had the highest discriminatory ability, the highest C-index value. NIACE score had the lowest akaike information criterion value. In this French cohort, BCLC nomogram and NIACE score provided the best prognostic information.
- Citation: Adhoute X, Pénaranda G, Raoul JL, Edeline J, Blanc JF, Pol B, Campanile M, Perrier H, Bayle O, Monnet O, Beaurain P, Muller C, Castellani P, Le Treut YP, Bronowicki JP, Bourlière M. Barcelona clinic liver cancer nomogram and others staging/scoring systems in a French hepatocellular carcinoma cohort. World J Gastroenterol 2017; 23(14): 2545-2555
- URL: https://www.wjgnet.com/1007-9327/full/v23/i14/2545.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i14.2545
Survival prediction and therapeutic strategy for hepatocellular carcinoma (HCC) are based on Barcelona classification of liver cancer staging system (BCLC) in the West[1,2]. It has become the reference classification by its prognostic value, its simplicity, and its treatment algorithm based on randomized clinical studies[3]. However, HCC staging systems remain a controversial issue. Asian countries, in which HCC is mainly related to HBV, have their own staging systems and therapeutic recommendations[4]. The BCLC system has been criticized; the major issue is that stages B and C HCC include a broad spectrum of tumors with a single therapeutic option[5-7], and for some authors other treatments are possible[8-11]. Subsequently, changes have been made compared to the initial version of the BCLC system[12] with the transfer of single and large HCC > 50 mm from intermediate to early stages[3], enhancing the heterogeneity within this group[13]. Older scores such as CLIP[14] showed a better prognostic value than the BCLC system in large Asian and Western HCC cohorts[15,16]. Therefore, a new classification has been proposed, the HKLC system[17], which offers another stratification, and new therapeutic proposals with surgery and chemoembolization to treat more advanced HCC. Other scores, independent of the BCLC system[7,18,19] or additional to the BCLC system[20,21] have been proposed in recent years. NIACE score (tumor Nodularity, Infiltrative nature of the tumor, serum Alpha-fetoprotein level, Child-Pugh stage, ECOG performance status)[22] determines sub-groups of different survival prognosis irrespective of the BCLC stage[23], or HCC treatment modalities[24]. This score has been validated either in European or Asian cohorts[25,26]. Recently, Hsu et al[27] proposed a simple nomogram, determined from a large HCC cohort mainly related to HBV in order to improve the prognostic value of the BCLC system.
The aims of this study were to assess and compare the performances of the BCLC nomogram and others staging and scoring systems (BCLC, HKLC, CLIP and NIACE) for survival prediction in a large European multicenter HCC cohort.
This retrospective study was conducted in five French centers (Marseille, Nancy, Bordeaux, and Rennes). During a period of seven years, from January 2007 to December 2013, all HCC patients treated or not, have been included in this study.
HCC diagnosis was based on the identification of the typical hallmark of HCC (EASL - AASLD criteria)[28] and, if a patient did not have a typical HCC on imaging or a cirrhotic liver, or if there was discordant results between non-invasive criteria (such as fibrometer and fibroscan), a biopsy was required. The analyzed data (clinical, biological, radiological, therapeutic options, response to treatment and follow-up) were prospectively collected and retrospectively analyzed using the same methodology in the different centers. This study was approved by local ethics committee.
HCC were ranked at diagnosis and during follow-up according to their morphologies (nodular or infiltrative HCC) assessed by multi-sliced contrast-enhanced CT and/or MRI. Liver cancers were either nodular HCC, that is an arterially enhancing mass with clear demarcation and washout in the portal venous phase, or infiltrative HCC, that is an ill-defined tumor with no distinct margination of any portion, characterized by inhomogeneous areas of enhancement on the arterial phase images and corresponding areas of washout on more delayed phases of contrast enhancement. These tumors may be more visible among the surrounding liver parenchyma at diffusion- and T2- weighted MR images and are frequently associated with vascular invasion[29-32]. Early (BCLC A) and intermediate (BCLC B) HCC without vascular invasion, considered as infiltrative tumor as opposed to encapsulated tumors, were tumor with non-smooth tumor margins (i.e., tumor with focal extranodular extension beyond the tumor capsule or focal infiltrative margin), or those with peritumoral enhancement[33-35], or those associated with biliary dilatation. Two liver imaging “senior experts” radiologists reviewed images retrospectively.
Following categories were used for the BCLC classification: BCLC A HCC was defined as patients having solitary tumor > 2 cm or no more than 3 tumors not exceeding 3 cm in diameter, PS 0, Child-Pugh grade A or B.
BCLC B HCC encompassed patients with multiple tumors beyond 3 cm, PS 0, Child-Pugh grade A or B.
BCLC C encompassed any tumor with radiologically evident or histologically proven macrovascular invasion (portal vein, hepatic vein, inferior vena cava) and/or patients with lymph nodes and/or distant metastases and/or patients with cancer related - symptoms, with preserved liver function.
BCLC D encompassed tumors leading to a very poor performance status (PS 3-4), or patients with severe liver impairment (Child-Pugh B9 grade) and tumors beyond the transplantation threshold. Child-Pugh C patients were excluded because the NIACE score did not incorporate Child-Pugh C grade.
The HKLC classification, the CLIP score and the BCLC nomogram were applied to each patient before treatments initiation.
The NIACE score was calculated with all parameters collected before treatments initiation, as follows: 1x (Nodular numbers 0 if < 3, 1 if ≥ 3) + 1.5x (Infiltrating tumors: 0 if no, 1 if yes) + 1.5x (Alpha-fetoprotein level: 0 if < 200, 1 if ≥ 200 ng/mL) + 1.5x (Child-Pugh grade: 0 if A, 1 if B) + 1.5x (ECOG PS score 0 if 0, 1 if ≥ 1).
Treatment and follow-up modalities were applied similarly in all centers.
Surgery: In general, patients with resectable tumors were selected for surgery if they had a performance status of 0 with both Child-Pugh grade A or B7, and on the basis of their functional hepatic reserve (indocyanine green retention rate at 15 min < 15%) and on the estimated remnant liver volume, regardless of HCC morphologies. Our protocols for the assessment of FHR and determination of surgical extent include biochemical liver function tests, blood cell count, IGR R15, and triphasic liver CT with volumetry. Gastroesophageal endoscopic findings were also taken into consideration for cirrhotic livers.
Patients without clinically significant portal hypertension and with normal serum bilirubin value were first considered for resection. Patients who underwent surgery vs radiofrequency ablation were as expected younger with less cirrhosis and larger tumor size. In cirrhosis, candidates for resection were carefully selected to diminish the risk of post-operative liver failure[36]. Portal hypertension (presence of either esophageal varices (EV), or splenomegaly with platelet count below 100000/mm3) was considered as a contraindication for liver resection, but in BCLC A HCC patients with well-preserved liver function, and IGR at 15 min < 15%, not suitable for radiofrequency ablation (RFA) or transplantation, a minor hepatic resection was proposed[37-39]. Surgery was made after endoscopic treatment of EV.
Some BCLC C HCC patients were selected for hepatectomy according to the following selection criteria: PS 0, Child-Pugh A with bilirubin level ≤ 1.0 mg/dL, single nodule with limited portal vein thrombosis (i.e., with second-order branch and third-order branch)[8].
Radiofrequency ablation: Applied in patients with resectable tumor ≤ 50 mm of diameter or within the Milan criteria (single tumor ≤ 50 mm or up to three tumors ≤ 30 mm in diameter).
Patients who underwent both radiofrequency ablation and chemoembolization vs radiofrequency ablation alone had larger tumor size.
Chemoembolization: Multinodular HCC with enhancing lesions, PS 0, Child-Pugh grade A or B7, were treated by TACE, regardless of HCC morphologies. Patients were treated by conventional TACE using the same inclusion/exclusion criteria in the different centers. TACE (Trans Arterial Chemoembolization) was performed in a standard fashion with a selective injection of a mixture of epirubicin (50 mg) and lipiodol (10 mL), followed by embolization with Gelfoam fragments. A second TACE was carried out 6 to 8 weeks later unless clear progress or serious adverse events occurred. Other TACE procedures were planned “on demand”, according to the results of radiological and AFP assessments made every 12 wk. The EASL criteria, based on a bi-dimensional measurement of the tumor’s enhanced viable component, were used to evaluate tumor response[40,41].
Patients with segmental vein thrombosis were left in the analysis because, in most centers, this is not considered as a contraindication for TACE[42,43].
Patients excluded from this retrospective analysis were: patients who received TACE as a bridge for liver transplantation; Child-Pugh C patients, and patients treated by liver transplantation.
The initial sorafenib dose was determined according to different factors, such as Eastern Cooperative Oncology Group Performance Status and liver function. Child-Pugh A patients received 400 mg twice a day and Child-Pugh B patients 200 mg, twice a day. A reduction in the sorafenib dose or a temporary interruption was allowed, depending on the type and severity of any adverse event (grade 2 or higher on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 4.0). Sorafenib treatment was continued unless intolerable toxicity or clinical disease progression was observed. CT and/or MRI were used to evaluate the tumor response every 3 mo.
Patients had received Sorafenib since 2008; fifty six patients had received other palliative treatments before 2008 including tamoxifen or pravastatin (n = 23), or chemotherapy with doxorubicin (n = 20), and others drugs in clinical trials (n = 13).
Continuous data are expressed as median [quartile 1 - quartile 3] and categorical data are expressed as rates. Normality of the data was assessed by Shapiro-Wilks test. Overall survival was the endpoint used. The time of survival was defined as the time interval between the diagnosis of hepatocellular carcinoma and death or time of last follow-up. Proportionality of the subdistribution hazards was assessed by both inspecting Schoenfeld-type residuals and testing correlation of these residuals with time[44]. In case of proportionality of hazards across time, survivals between groups were compared using log-rank test; generalized Wilcoxon test was used in case on non-proportionality of hazards[45]. Discriminatory ability of each staging system was performed using χ² linear trend test (LT) and the Akaike information criteria (AIC): the higher is the LT and the lower is the AIC, the higher is the discriminatory ability of the model. Homogeneity of each staging system was performed using likelihood ratio (LR) calculated using the Cox regression model: the higher de LR, the lower is the difference among the patients classified into the same group by each staging system. The C-index was also used to determine the performance of the model. The larger the C-index, the more accurate the prognostic prediction was[46]. All p-values were considered significant at α-level = 0.05. All calculations were performed using the SAS V9.1 statistical software (SAS Institute Inc. Cary, NC).
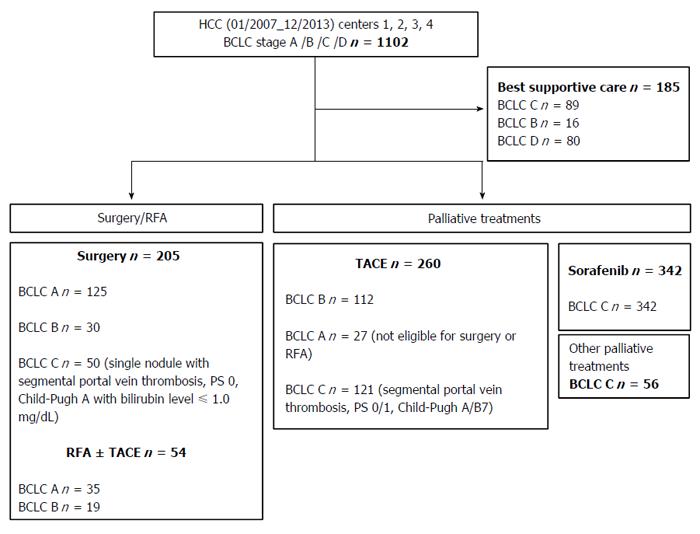
Patients’ characteristics are indicated in Table 1. The cohort included a total of 1102 patients, the majority of patients were male (86%) and the median age was 68 [60-74] years. Cirrhosis was present in 81% of patients; 73% of them were ranked Child-Pugh grade A. Underlying liver disease was related to alcohol in 41% of the patients, and to viral C hepatitis in 27% of the patients. HCC were multinodular in 59% of the cases and 43% of the patients had at least three nodules. Portal vein thrombosis was present in 41% of the cases, and 43% of HCCs were infiltrating tumors. Baseline ECOG performance status of our population (as expression of symptomatic tumor) was as follows: PS 0 (50%), PS 1-2 (46%), PS 3-4 (4%).
| All patients (n = 1102) | |
| Age - Median (Q1-Q3), yr | 68 (60-74) |
| Gender | |
| Male/Female | 943 (86)/159 (14) |
| Liver disease | |
| Alcoholism/HCV/HBV/MS/ Other | 452 (41)/297 (27)/66 (6)/99 (9)/188 (17) |
| Cirrhosis | 895 (81) |
| Child - Pugh grade | |
| A/B | 653 (73)/242 (27) |
| Tumor Size (Q1-Q3) mm | 43 (20-75) |
| Multifocal | 654 (59) |
| Nodules | |
| < 3/≥ 3 | 633 (57)/469 (43) |
| Portal vein thrombosis | 452 (41) |
| Infiltrative HCC | 469 (43) |
| AFP - Median [Q1-Q3], ng/mL | 53 (7-1300) |
| ECOG (PS) | |
| 0/1-2/3-4 | 553 (50)/506 (46)/43 (4) |
| BCLC stage | |
| A/B/C/D | 187 (17)/177 (16)/658 (60)/80 (7) |
| Treatment allocation | |
| Resection/RFA ± TACE | 259 (23.5) |
| TACE | 260 (23.5) |
| Sorafenib | 342 (31) |
| Other palliative treatments | 56 (5) |
| Supportive care | 185 (17) |
The stratification of patients according to the BCLC system was as follows: BCLC A (17%), BCLC B (16%), BCLC C (60%), and BCLC D (7%).
The primary anti-cancer treatments of patients are shown in Figure 1 and Table 1. twenty-three point five percent of the patients received treatments of curative intent (surgery, RFA ± TACE), while 59.5% of the patients received a palliative treatment (TACE, sorafenib, others systemic treatments) and 17% only best supportive care.
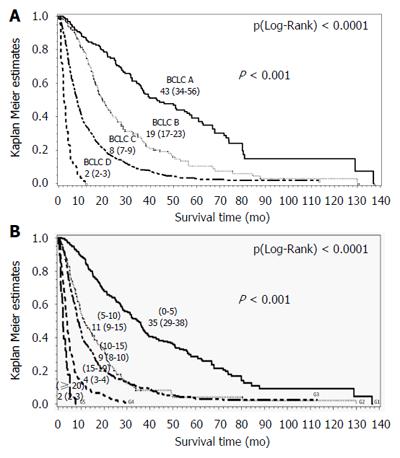
Median overall survival for the entire cohort was 10.8 mo [4.9-28.0], consistent with the median follow-up duration: 10 mo [4.4-22.7]. Eighty-two percent of patients died. Median overall survival according to the BCLC system was as follows: BCLC A 43 mo [36-57], BCLC B 19 mo [17-23], BCLC C 8 mo [7-9] and BCLC D 2 mo [2-3] (P (Log-Rank) < 0.0001) (Figure 2A).
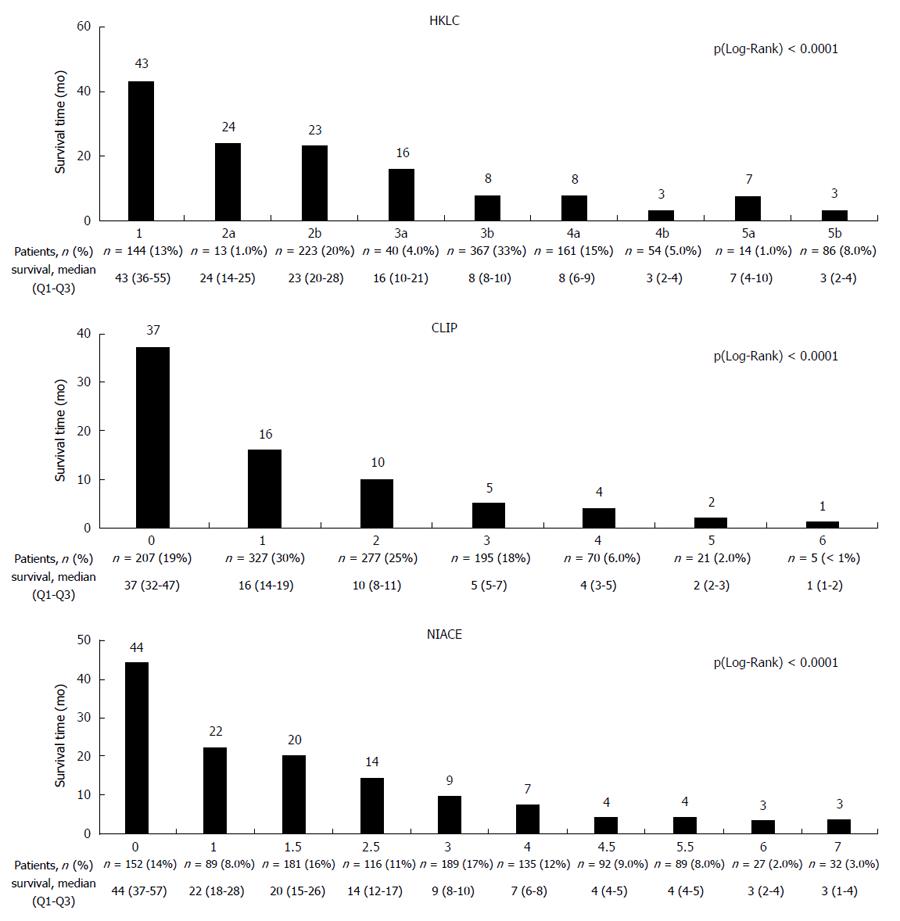
The HKLC system differentiated within this cohort between nine subgroups with median overall survival ranging from 43 [36-55] mo for the HKLC group 1 to 3 [2-4] mo for the HKLC group 5b, P (Wilcoxon) < 0.0001. However, several subgroups (IIa/IIb, IIIb/IVa, IVb/Vb) had a similar overall survival (Figure 3).
The CLIP and NIACE scores differentiated within this cohort seven and ten subgroups respectively with a different prognosis, P (Wilcoxon) < 0.0001 (Figure 3). CLIP scores ranked 74% of the patients in the first three groups (0 - 1 - 2): 19%, 30% and 25%, respectively. The distribution of patients in the ten subgroups from the NIACE score was more homogeneous (NIACE 0: 14%, 1: 8%, 1.5: 16%, 2.5: 11%, 3: 17%, 4: 12%, 4.5: 9%, 5.5: 8%, 6: 2% and NIACE 7: 3%).
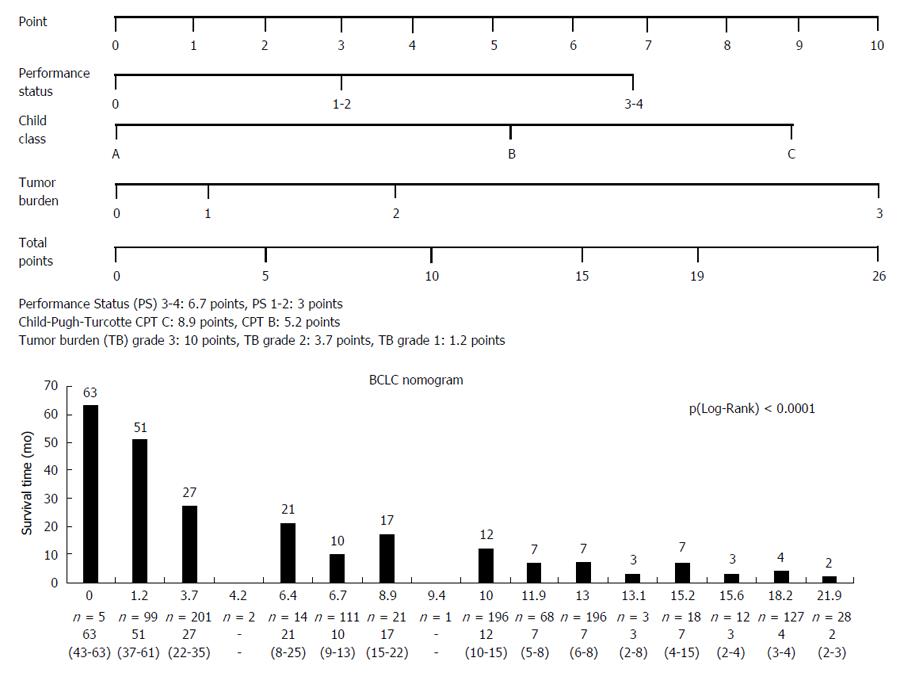
The nomogram values within the cohort are shown in the Figure 4. In summary, the nomogram distinguished sixteen subgroups. Analysis of survival time based on nomogram BCLC values showed a significant difference, P (Wilcoxon) < 0.0001, survival time decreased with increasing nomogram values.
Performances of the nomogram and other staging and scoring systems for survival prediction are indicated in Table 2. The C-index of the nomogram for predicting overall survival was 0.719, significantly higher than the BCLC system (0.674), the HKLC system (0.698). The nomogram yielded a higher discriminative ability (LT (χ²) = 93.2169) than the other systems. The likelihood ratio test showed that the nomogram had an additional homogeneity of survival within each score (500.7218) close to the best value produced by the NIACE score (532.0369), and higher than other systems. Moreover, the nomogram was associated with a lower corrected Akaike information criterion (10679.513) compared with the other systems and close to the best value produced by the NIACE score (10648.198).
| Score | Discriminatory ability linear trend test | Homogeneity likelihood ratio test | Akaike information criterion | C-index | ||
| LT (χ²) | P value | LR (χ²) | P value | |||
| BCLC Nomogram | 93.2169 | < 0.0001 | 500.7218 | < 0.0001 | 10679.513 | 0.719 |
| NIACE | 91.6906 | < 0.0001 | 532.0369 | < 0.0001 | 10648.198 | 0.718 |
| BCLC | 79.0342 | < 0.0001 | 380.4100 | < 0.0001 | 10805.825 | 0.674 |
| HKLC | 71.8861 | < 0.0001 | 455.3169 | < 0.0001 | 10740.918 | 0.698 |
| CLIP | 87.2785 | < 0.0001 | 430.3872 | < 0.0001 | 10749.848 | 0.716 |
| Nomogram according to BCLC last version | 86.1320 | < 0.0001 | 417.4356 | < 0.0001 | 10762.799 | 0.698 |
Our findings indicate that the nomogram has a good stratification ability with regard to prognosis in patients with HCC, within a European HCC cohort, mostly BCLC-C[47,48] compared to other known staging and scoring systems (BCLC, HKLC systems, CLIP score). By specifying the magnitude of each variable within the BCLC system (tumor burden, liver function, general conditions), the nomogram can better predict the survival of patients with HCC. In previous studies, CLIP and NIACE scores showed a better predictive value for survival compared to other staging and scoring systems within two large Asian and European HCC cohorts[15,25,26].
In our study the CLIP score also distinguished between subgroups with significantly different survival, but the majority of patients (74%) were in the first three groups (CLIP 0, 1 and 2), as previously described[15,49,50], limiting its discriminatory capacity.
The HKLC classification proposes another stratification with five groups and nine subgroups in order to enhance prognostic accuracy for HCC; the early stages (I, IIa) include BCLC A and B HCC patients, the intermediate stages (IIb, IIIa) include BCLC A, B and C HCC patients and the locally advanced stages (IIIb) include BCLC B and C HCC patients. Despite a greater number of subgroups, some of them had the same survival (IIa/IIb, IIIb/IVa and IVb/Vb), as previously reported[51], reducing the usefulness of this new classification in a European cohort.
The nomogram showed a higher predictive power for survival within this external European cohort, but there is still some issue. The nomogram is a reliable predictor of survival for patients with HCC, however this nomogram is complex ranging from 0 to 26 points and in our cohort, it distinguished sixteen subgroups. Moreover, it doesn’t help clinicians in treatment decision. A simplified stratification into five sub-groups is possible: [0-5], [5-10], [10-15], [15-19], and [≥ 20]; the survival time observed in our cohort was respectively: 35 [30-38] mo, 12 [10-16] mo, 9 [8-10] mo, 4 [3-4] mo, and 2 [2-3] mo, P < 0.0001 (Figure 2B). These results should be validated, or other thresholds may be suggested by a specific analysis.
There is another issue with the nomogram after the adoption of changes in the BCLC system[3], which could affect its discriminatory capacity. Single and large tumors (> 50 mm) were included into the BCLC A group; therefore, they should logically be included in the tumor burden grade 1 and not 2. By applying this rule, the predictive value of the nomogram became lower (c-index: 0.698 vs 0.719) (Table 2).
In addition, the prognostic accuracy of the nomogram and the NIACE are close within this cohort. However, NIACE score is not only an additional prognostic score to the BCLC system[22,26], but it can be used as an aid to the decision-making process, distinguishing different prognostic groups among patients treated by surgery or those treated by TACE or Sorafenib[22,24]. The combination of classification plus scores (BCLC and NIACE) have already showed an additional value for treatment recommendation in a retrospective cohort and prospective validation study should be designed[52].
There are several limitations of the present study including the retrospective study design, its multicenter nature, which may make bias unavoidable. Regarding treatment decision, BCLC treatment recommendations are seldom followed due to great heterogeneity within each stage[48,53,54]. In our study, 33% of patients received treatment outside BCLC recommendations [14% of BCLC A HCC patients (n = 27), 28% of BCLC B HCC patients (n = 49), and 40% of BCLC C HCC patients (n = 227)]. sixty-two percent of patients undergoing surgery or RFA were ranked as BCLC A HCC, 43% of patients treated by TACE were ranked as BCLC B HCC, and 40% of treated BCLC C patients received a first-line treatment other than sorafenib. Our cohort mainly included advanced HCC, that is a heterogeneous population with limited therapeutic option until now, namely sorafenib with modest survival benefit[55] or inclusion in randomized trials who do not reflect patients in daily clinical practice. In our study like others[56-59] impairment of liver function is the major factors that preclude patient to receive sorafenib. Moreover BCLC-C patients before sorafenib availability have received others non-valuable treatment. Each BCLC stage including a broad spectrum of tumors, a proportion of patients in each stage do not fulfill all the criteria for the treatment allocation, and for some authors other therapeutic options are possible[8,54,60,61]. Therefore treatment recommendations based on new combination of BCLC and scoring systems such as NIACE or other are urgently required.
In summary, this study confirms the BCLC nomogram as a new HCC reliable prognostic tool; its predictive value on survival is higher compared to known classifications and scoring system. However, the usefulness of this nomogram is limited due to its complexity and the fact that it is not linked to a therapeutic strategy. BCLC system remains the most widely used staging system, however BCLC treatment recommendations are seldom followed suggesting the need for better tools.
Hepatocellular carcinoma (HCC) prognosis is still a controversial issue. Barcelona Clinic Liver Cancer staging system has limits [heterogeneity of the Barcelona clinic liver cancer (BCLC) subgroups, strict therapeutic algorithm]. Using a nomogram as proposed by Hsu et al to improve BCLC system prognostic value is an attractive idea for clinicians.
Hsu et al think that conferring value on each of the three main parameters of the BCLC system ie tumor burden, liver function and performance status (using a multivariate Cox regression model within a large Asian HCC cohort), could improve the individual prognosis of HCC patients. The authors think that prognosis and treatment of HCC should be associated. They assessed the reliability and the usefulness of the BCLC nomogram within a European cohort mainly related to alcohol abuse and HCV hepatitis.
This paper shows that the BCLC nomogram is a reliable tool for HCC prognosis, irrespective of the underlying liver disease, with a better predictive value for survival compared to other scoring or staging systems (CLIP, HKLC). But its usefulness is limited by its complexity (tumor burden grade 3: 10 points, grade 2 and 1: 3.7 and 1.2 points; Child-Pugh grade C: 8.9 points, Child-Pugh grade B and A: 5.2 and 0 points; PS 3-4: 6.7 points, PS 1-2 and 0: 3 and 0 points) and the lack of therapeutic link. They Suggest an additional score (including other prognostic variables such as AFP serum level and/or tumor morphology) to the BCLC system in order to improve the prognostic information and the therapeutic decision.
BCLC nomogram provides reliable prognostic information for HCC patients, irrespective of underlying liver disease, but it doesn’t guide the therapeutic decision. Conversely a combination of BCLC system and scores may influence HCC prognosis and its therapeutic management.
NIACE score (tumor Nodularity, Infiltrative nature of the tumor, serum Alpha-fetoprotein level, Child-Pugh stage, ECOG performance status) determines sub-groups of different survival prognosis irrespective of the BCLC stage, or HCC treatment modalities.
The aim of this study is to compare the performances of several HCC staging systems including the BCLC nomogram in the prediction of survival of a large French HCC cohort. A total of 1102 HCC patients retrospectively recruited from 5 hospitals in different areas. The objective of this study is clear and the statistical studies were well done. The conclusion is logical and adequate.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hsieh SY S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] [Cited in This Article: ] |
| 2. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4333] [Cited by in F6Publishing: 4429] [Article Influence: 233.1] [Reference Citation Analysis (0)] |
| 3. | European Association For The Study Of The Liver1; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4399] [Article Influence: 366.6] [Reference Citation Analysis (2)] |
| 4. | Han KH, Kudo M, Ye SL, Choi JY, Poon RT, Seong J, Park JW, Ichida T, Chung JW, Chow P. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81 Suppl 1:158-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 6. | Lee S, Kim BK, Song K, Park JY, Ahn SH, Kim SU, Han KH, Kim do Y. Subclassification of Barcelona Clinic Liver Cancer B and C hepatocellular carcinoma: A cohort study of the multicenter registry database. J Gastroenterol Hepatol. 2016;31:842-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Yau T, Yao TJ, Chan P, Ng K, Fan ST, Poon RT. A new prognostic score system in patients with advanced hepatocellular carcinoma not amendable to locoregional therapy: implication for patient selection in systemic therapy trials. Cancer. 2008;113:2742-2751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 395] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 9. | Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD, Luque A, Zurera L, Espejo JJ, Rodríguez-Perálvarez M. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015;41:1153-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Pai JT, Loong CC, Chiou YY, Lee RC, Lee FY. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol. 2012;19:842-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 12. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2645] [Cited by in F6Publishing: 2784] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 13. | Liu PH, Su CW, Hsu CY, Hsia CY, Lee YH, Huang YH, Lee RC, Lin HC, Huo TI. Solitary Large Hepatocellular Carcinoma: Staging and Treatment Strategy. PLoS One. 2016;11:e0155588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 908] [Cited by in F6Publishing: 941] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 15. | Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, Lee FY, Lin HC, Huo TI. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol. 2016;64:601-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 16. | Collette S, Bonnetain F, Paoletti X, Doffoel M, Bouché O, Raoul JL, Rougier P, Masskouri F, Bedenne L, Barbare JC. Prognosis of advanced hepatocellular carcinoma: comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 502] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 18. | Hucke F, Pinter M, Graziadei I, Bota S, Vogel W, Müller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatol. 2014;61:1287-1296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Su CW, Lee FY, Lin HC, Huo TI. Proposal and validation of a new model to estimate survival for hepatocellular carcinoma patients. Eur J Cancer. 2016;63:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Müller C, Heinzl H, Trauner M, Peck-Radosavljevic M. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261-2273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 21. | Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, Monnet O, Beaurain P, Bazin C, Pol B. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015;62:855-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Adhoute X, Pénaranda G, Raoul JL, Blanc JF, Edeline J, Conroy G, Perrier H, Pol B, Bayle O, Monnet O. Prognosis of advanced hepatocellular carcinoma: a new stratification of Barcelona Clinic Liver Cancer stage C: results from a French multicenter study. Eur J Gastroenterol Hepatol. 2016;28:433-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Adhoute X, Penaranda G, Blanc JF, Edeline J, Naude S, Perrier H, Monnet O, Castellani P, Oules V, Bayle O. Stratification of hepatocellular carcinoma. The prognostic score NIACE, an additional aid to the Barcelona Clinic Liver Cancer (BCLC) staging system? Multicenter study. J Hepatol. 2015;62 Suppl 2:S453. [DOI] [Cited in This Article: ] |
| 24. | Su TH, Liu CJ, Yang HC, Liu CH, Chen PJ, Chen DS, Adhoute X, BourliereM , Kao JH. The NIACE score helps predict the survival of Asian hepatocellular carcinoma patients. J Gastroenterol Hepatol. 2015;30:23. [Cited in This Article: ] |
| 25. | Adhoute X, Penaranda G, Raoul JL, Bourlière M. Hepatocellular carcinoma scoring and staging systems. Do we need new tools? J Hepatol. 2016;64:1449-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Liu PH, Huo TI. Reply to “Hepatocellular carcinoma scoring and staging systems. Do we need new tools?”. J Hepatol. 2016;64:1450-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Hsu CY, Liu PH, Hsia CY, Lee YH, Al Juboori A, Lee RC, Lin HC, Huo TI. Nomogram of the Barcelona Clinic Liver Cancer system for individual prognostic prediction in hepatocellular carcinoma. Liver Int. 2016;36:1498-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6408] [Article Influence: 492.9] [Reference Citation Analysis (1)] |
| 29. | Rosenkrantz AB, Lee L, Matza BW, Kim S. Infiltrative hepatocellular carcinoma: comparison of MRI sequences for lesion conspicuity. Clin Radiol. 2012;67:e105-e111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Reynolds AR, Furlan A, Fetzer DT, Sasatomi E, Borhani AA, Heller MT, Tublin ME. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics. 2015;35:371-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Kim YK, Han YM, Kim CS. Comparison of diffuse hepatocellular carcinoma and intrahepatic cholangiocarcinoma using sequentially acquired gadolinium-enhanced and Resovist-enhanced MRI. Eur J Radiol. 2009;70:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Kanematsu M, Semelka RC, Leonardou P, Mastropasqua M, Lee JK. Hepatocellular carcinoma of diffuse type: MR imaging findings and clinical manifestations. J Magn Reson Imaging. 2003;18:189-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Shimada M, Rikimaru T, Hamatsu T, Yamashita Y, Terashi T, Taguchi K, Tanaka S, Shirabe K, Sugimachi K. The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am J Surg. 2001;182:177-182. [PubMed] [Cited in This Article: ] |
| 34. | Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, Brandi G, Pinna AD, Golfieri R. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma? Radiology. 2016;279:432-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 35. | Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, Choi JS, Han KH, Kim E, Kim KW. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009;19:1744-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Choi SB, Kim HJ, Song TJ, Ahn HS, Choi SY. Influence of clinically significant portal hypertension on surgical outcomes and survival following hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2014;21:639-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Capussotti L, Ferrero A, Viganò L, Muratore A, Polastri R, Bouzari H. Portal hypertension: contraindication to liver surgery? World J Surg. 2006;30:992-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 39. | Zhong JH, Li H, Xiao N, Ye XP, Ke Y, Wang YY, Ma L, Chen J, You XM, Zhang ZY. Hepatic resection is safe and effective for patients with hepatocellular carcinoma and portal hypertension. PLoS One. 2014;9:e108755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 41. | Kim BK, Kim KA, Park JY, Ahn SH, Chon CY, Han KH, Kim SU, Kim MJ. Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolisation. Eur J Cancer. 2013;49:826-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, Stauber R, Grünberger B, Müller C, Kölblinger C. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 44. | Kohl M, Plischke M, Leffondré K, Heinze G. PSHREG: a SAS macro for proportional and nonproportional subdistribution hazards regression. Comput Methods Programs Biomed. 2015;118:218-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 45. | Peto R, Pike MC. Conservatism of the approximation sigma (O-E)2-E in the logrank test for survival data or tumor incidence data. Biometrics. 1973;29:579-584. [PubMed] [Cited in This Article: ] |
| 46. | Huitzil-Melendez FD, Capanu M, O’Reilly EM, Duffy A, Gansukh B, Saltz LL, Abou-Alfa GK. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28:2889-2895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 47. | Adhoute X, Penaranda G, Raoul JL, Bourlière M. Nomogram of the Barcelona Clinic Liver Cancer System: external validation in European patients. Liver Int. 2016;36:1716-1717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 827] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 49. | Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 50. | Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 51. | Adhoute X, Penaranda G, Bronowicki JP, Raoul JL. Usefulness of the HKLC vs. the BCLC staging system in a European HCC cohort. J Hepatol. 2015;62:492-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Adhoute X, Penaranda G, Raoul JL, Bourlière M. HCC classification and HCC scoring system: a win-win combination for prognosis and treatment recommendations. Liver Int. 2016;36:1876-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Kim KM, Sinn DH, Jung SH, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. The recommended treatment algorithms of the BCLC and HKLC staging systems: does following these always improve survival rates for HCC patients? Liver Int. 2016;36:1490-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, Volk M, Giannini EG, Ciccarese F, Piscaglia F. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015;62:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 55. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9819] [Article Influence: 613.7] [Reference Citation Analysis (1)] |
| 56. | Wörns MA, Weinmann A, Pfingst K, Schulte-Sasse C, Messow CM, Schulze-Bergkamen H, Teufel A, Schuchmann M, Kanzler S, Düber C. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol. 2009;43:489-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 57. | Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 58. | Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 59. | Zugazagoitia J, Manzano A, Sastre J, Ladero JM, Puente J, Díaz-Rubio E. Sorafenib for non-selected patient population with advanced hepatocellular carcinoma: efficacy and safety data according to liver function. Clin Transl Oncol. 2013;15:146-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Hsu CY, Liu PH, Hsia CY, Lee YH, Nagaria TS, Lee RC, Lin HC, Huo TI. Surgical Resection is Better than Transarterial Chemoembolization for Patients with Hepatocellular Carcinoma Beyond the Milan Criteria: A Prognostic Nomogram Study. Ann Surg Oncol. 2016;23:994-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |