Copyright
©The Author(s) 2016.
World J Gastroenterol. Mar 7, 2016; 22(9): 2844-2854
Published online Mar 7, 2016. doi: 10.3748/wjg.v22.i9.2844
Published online Mar 7, 2016. doi: 10.3748/wjg.v22.i9.2844
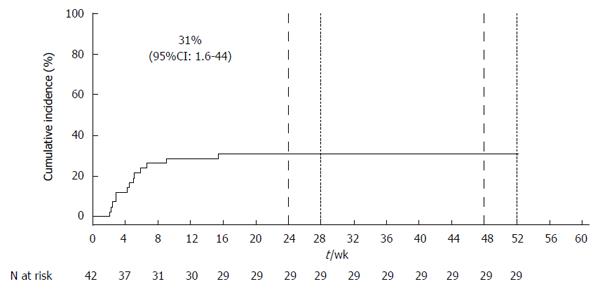
Figure 1 Cumulative incidence of hepatic decompensation and/or serious adverse events using the Kaplan-Meier method.
The dashed line indicates the time at which 24-wk and 48-wk treatment regimens were completed. The dotted line indicates the 4-wk post end of treatment surveillance period.
- Citation: Patel N, Bichoupan K, Ku L, Yalamanchili R, Harty A, Gardenier D, Ng M, Motamed D, Khaitova V, Bach N, Chang C, Grewal P, Bansal M, Agarwal R, Liu L, Im G, Leong J, Kim-Schluger L, Odin J, Ahmad J, Friedman S, Dieterich D, Schiano T, Perumalswami P, Branch A. Hepatic decompensation/serious adverse events in post-liver transplantation recipients on sofosbuvir for recurrent hepatitis C virus. World J Gastroenterol 2016; 22(9): 2844-2854
- URL: https://www.wjgnet.com/1007-9327/full/v22/i9/2844.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i9.2844