Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2533
Peer-review started: July 1, 2015
First decision: September 29, 2015
Revised: October 17, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: February 28, 2016
Processing time: 242 Days and 3.1 Hours
AIM: To investigate the effect of different dietary fatty acids on hepatic inflammasome activation.
METHODS: Wild-type C57BL/6 mice were fed either a high-fat diet or polyunsaturated fatty acid (PUFA)-enriched diet. Primary hepatocytes were treated with either saturated fatty acids (SFAs) or PUFAs as well as combined with lipopolysaccharide (LPS). The expression of NOD-like receptor protein 3 (NLRP3) inflammasome, peroxisome proliferator-activated receptor-γ and nuclear factor-kappa B (NF-κB) was determined by real-time PCR and Western blot. The activity of Caspase-1 and interleukine-1β production were measured.
RESULTS: High-fat diet-induced hepatic steatosis was sufficient to induce and activate hepatic NLRP3 inflammasome. SFA palmitic acid (PA) directly activated NLRP3 inflammasome and increased sensitization to LPS-induced inflammasome activation in hepatocytes. In contrast, PUFA docosahexaenoic acid (DHA) had the potential to inhibit NLRP3 inflammasome expression in hepatocytes and partly abolished LPS-induced NLRP3 inflammasome activation. Furthermore, a high-fat diet increased but PUFA-enriched diet decreased sensitization to LPS-induced hepatic NLRP3 inflammasome activation in vivo. Moreover, PA increased but DHA decreased phosphorylated NF-κB p65 protein expression in hepatocytes.
CONCLUSION: Hepatic NLRP3 inflammasome activation played an important role in the development of non-alcoholic fatty liver disease. Dietary SFAs and PUFAs oppositely regulated the activity of NLRP3 inflammasome through direct activation or inhibition of NF-κB.
Core tip: Our research inventively elucidated that high-fat diet-induced hepatic steatosis was sufficient to induce and activate hepatic NOD-like receptor protein 3 (NLRP3) inflammasome, playing an important role in the development of non-alcoholic fatty liver disease. Furthermore, dietary saturated fatty acids and polyunsaturated fatty acids oppositely regulated the activity of NLRP3 inflammasome in hepatocytes and transmitted different sensitization to lipopolysaccharide-induced activation of NLRP3 inflammasome in vitro and in vivo, partly through direct activation or inhibition of nuclear factor-kappa B.
- Citation: Sui YH, Luo WJ, Xu QY, Hua J. Dietary saturated fatty acid and polyunsaturated fatty acid oppositely affect hepatic NOD-like receptor protein 3 inflammasome through regulating nuclear factor-kappa B activation. World J Gastroenterol 2016; 22(8): 2533-2544
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2533.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2533
Nonalcoholic fatty liver disease (NAFLD) is considered the hepatic manifestation of the metabolic syndrome. The spectrum of NAFLD includes simple steatosis, steatosis with inflammation and non-alcoholic steatohepatitis (NASH) with fibrosis. A “two hit” mechanism is proposed to drive progression from NAFLD to NASH. The initial step involves excessive fat accumulation in the liver and the second hit includes enhanced lipid peroxidation and increased generation of reactive oxygen species (ROS). Subsequently, fat accumulation in the liver enhances sensitization to the second hit or further insults. Recently, increasing evidence has suggested chronic and systemic low-grade inflammation play an essential role in the pathogenesis of insulin resistance and NAFLD[1,2].
Inflammasome has emerged as an important regulator of inflammation[3,4]. The NOD-like receptor protein 3 (NLRP3) inflammasome is a cytosolic protein complex composed of the regulatory subunit NLRP3, the adaptor apoptosis-associated speck-like protein (ASC) and the effector caspase-1. Inflammasome can be activated by both pattern-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs)[5,6]. The two-signal hypothesis is accepted in inflammasome activation: the first signal produces and induces pro-interleukine-1 beta (pro-IL-1β) and inflammasome components expression, and the second signal assembles inflammasome components to engage in caspase-1 activation and secret bioactive IL-1β.
Recent studies suggest a critical role for NLRP3 inflammasome in obesity and its complications[7-9]. Loss of NLRP3 inflammasome components protects mice against insulin resistance and metabolic dysfunction, indicating that NLRP3 inflammasome may function as a sensor to detect metabolic danger signals. Free fatty acids (FFAs), usually elevated in plasma of NASH patients[10,11], have been proposed as a major contributing factor for inflammatory response through engaging toll-like receptors (TLRs) and inducing nuclear factor-kappa B (NF-κB)-dependent production of inflammatory cytokines such as Tumor Necrosis Factor-α (TNF-α) and IL-6[12]. Recently, several studies have demonstrated that the saturated FFA (SFA) palmitic acid triggered inflammation by activating inflammasome in macrophages and hepatocytes[13,14]. However, these studies indicated SFAs alone only primed and induced the NLRP3 inflammasome expression but failed to completely activate NLRP3 inflammasome. Furthermore, there was no inflammasome activation in liver with simple hepatic steatosis in ob/ob mice. Therefore, the effect of SFAs or high-fat diet (HFD) on activation of the NLRP3 inflammasome needs to be further confirmed. Interestingly, recent studies revealed that n-3 polyunsaturated fatty acids (n-3 PUFAs) suppressed both lipopolysaccharide (LPS)-induced priming and NLRP3 inflammasome activation in macrophages and, more importantly, n-3 PUFAs prevented HFD-induced metabolic disorders such as insulin resistance through inhibition of NLRP3 inflammasome activation[15,16]. These results indicated that different types of fatty acids exerted different or even opposite roles in the activation of the NLRP3 inflammasome. Currently, the mechanism involved in inflammasome regulation remains largely unknown. Elevated ROS levels, mitochondria damage and lysosomal rupture have been proposed to participate in NLRP3 inflammasome activation[17-19] but little is known about FFAs to positively or negatively regulate NLRP3 inflammasome activation.
In the current study, we investigated whether HFD-induced hepatic steatosis had a direct effect on NLRP3 inflammasome activation. Furthermore, the effect of dietary SFAs and PUFAs on the NLRP3 inflammasome activation in hepatocytes was determined and the possible underlying mechanism was explored. We also determined the effect of different FFAs on LPS-induced NLRP3 inflammasome activation.
Adult (age 6-8 wk) male wild type C57BL/6 mice were obtained from the Experimental Animal Center (Ren Ji Hospital, Shanghai Jiao Tong University). Mice were fed either a normal control diet (NCD, 12% kcal from fat, n = 10) or a high-fat diet (HFD, 59% kcal from fat, Shanghai SLACCAS Company, n = 10) for 14 wk. All mice were maintained in a temperature and light-controlled facility and permitted to consume water and pellet chow ad libitum. All animal experiments fulfilled Shanghai Jiao Tong University criteria for the humane treatment of laboratory animals and was approved by the Ren Ji Hospital Animal Care and Use Committee (SYXK 2011-0121).
For LPS-induced liver injury, mice received an intraperitoneal injection of lipopolysaccharide (LPS, 1.5 mg/kg, Sigma-Aldrich Corporation, St. Louis, MO, United States) 6 h before being sacrificed after feeding with either NCD (n = 5), HFD (n = 5) or PUFA-enriched diet (enriched in polyunsaturated fatty acid, 45% kcal from fish oil, Medicience Ltd, China, n = 5) for 4 wk. The serum and liver tissue were collected.
Hepatocytes were isolated as previously described[20] and obtained through low-speed centrifugation of the liver filtrate and cultured in DMEM solution containing 12% fetal bovine serum (FBS, Gibco, Grand Island, NY, United States), with 100 U/mL penicillin G and 100 U/mL streptomycin sulfate on collagen I-coated plates. The viability of cells was assessed by the trypan blue exclusion test. Exclusion was greater than 90% for hepatocytes.
After overnight culture, hepatocytes were treated with either palmitic acid (PA, 0.5 mmol/L, Sigma-Aldrich Corporation, St. Louis, MO, United States) or docosahexaenoic acid (DHA, 50 μmol/L, Sigma-Aldrich Corporation, St. Louis, MO, United States) for 6 or 24 h, as well as combined with LPS (1 μg/mL, Sigma-Aldrich Corporation, St. Louis, MO, United States) treatment. Meanwhile, DMEM solution or LPS treatment alone served as a control respectively in the separated experiments.
Total RNA was extracted from mice liver tissue and hepatocytes using TRIzol reagent (Invitrogen, Grand Island, NY, United States). cDNA was synthesized from 2 μg of total RNA using Primescript RT Reagent kit (TaKaRa, Japan). For real-time PCR, 10 ng template was added in a 10 μL reaction system containing each primer and SYBR Green PCR Master Mix (TaKaRa, Japan). PCR thermocycling parameters were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s performed by ABI Prism 7300 system (Applied Biosystems, Grand Island, NY, United States). All reactions were performed in triplicate. The expression levels of target genes were quantified by the double-delta method (2-ΔΔCt). Murine primers (provided by Sangon Biotech Co., Shanghai, China) were as follows: NLRP3: GAGTTCTTCGCTGCTATGT and ACCTTCACGTCTCGGTTC; Caspase-1: TGGAGAGAAACAAGGAG and TTGAAGAGCAGAAAGCAAT; IL-1β: TCTTTGAAGTTGACGGACCC and TGAGTGATACTGCCTGCCTG; TNF-α: TCTTCTCATTCCTGCTTGTGG and GGTCTGGGCCATAGAACTGA; PPARγ: GCCCTTTACCACAGTTGATTTCT and GTGATTTGTCCGTTGTCTTTCCT; β-actin: TGTTACCAACTGGGACGACA and CTGGGTCATCTTTTCACGGT.
Whole protein extracted from mice liver tissue and hepatocytes were resolved by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels according to standard procedures. The samples were then transferred to 0.45 μm nitrocellulose membranes (Bio-Rad, Hercules, CA, United States) and incubated at 4 °C overnight with antibodies against NLRP3 (1:1000, Abcam, Cambridge, MA, United States), Caspase-1 (procaspase-1 and Caspase-1-p10, 1:1000, Abcam, Cambridge, MA, United States), peroxisome proliferator-activated receptor-γ (PPAR-γ) (1:500, Cell Signaling Technology, Danvers, MA, United States), NF-κB p65 (1:1000, Cell Signaling Technology, Danvers, MA, United States), phosphorylated NF-κB p65 (1:500, Cell Signaling Technology, Danvers, MA, United States) and GAPDH (1:10000, KangChen Bio-tech, Shanghai, China). The blots were then incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (1:10000, KangChen Bio-tech, Shanghai, China) at room temperature for 1 h. Immunoreactive bands were detected with ECL Western blotting kit (Thermo Scientific Pierce, Grand Island, NY, United States), exposed to films and developed.
Protein from mice liver tissue and hepatocytes was extracted in the same way as above. The activity of Caspase-1 was assessed with the caspase-1 activity assay kit (BioVision, Milpitas, CA, United States) according to the manufacturer’s instruction.
Whole cell lysates were extracted from liver tissue. Cells culture supernatants of hepatocytes were collected after 24 h treatment. These lysates and culture supernatants were assayed for IL-1β production with IL-1β ELISA kit (eBioscience, San Diego, CA, United States) according to the manufacturer’s protocol.
Liver tissues were fixed in 10% formalin and embedded in paraffin. The sections were stained with hematoxylin-eosin and examined under Olympus light microscopy. Liver tissue and fatty acid treated hepatocytes were fixed in 10% formalin and stained with oil red O and hematoxylin for examination of lipid accumulation.
All statistical analyses were carried out with GraphPad Prism 5 and expressed as mean ± SEM. The paired-individual means were compared by t-test. P < 0.05 was considered statistically significant.
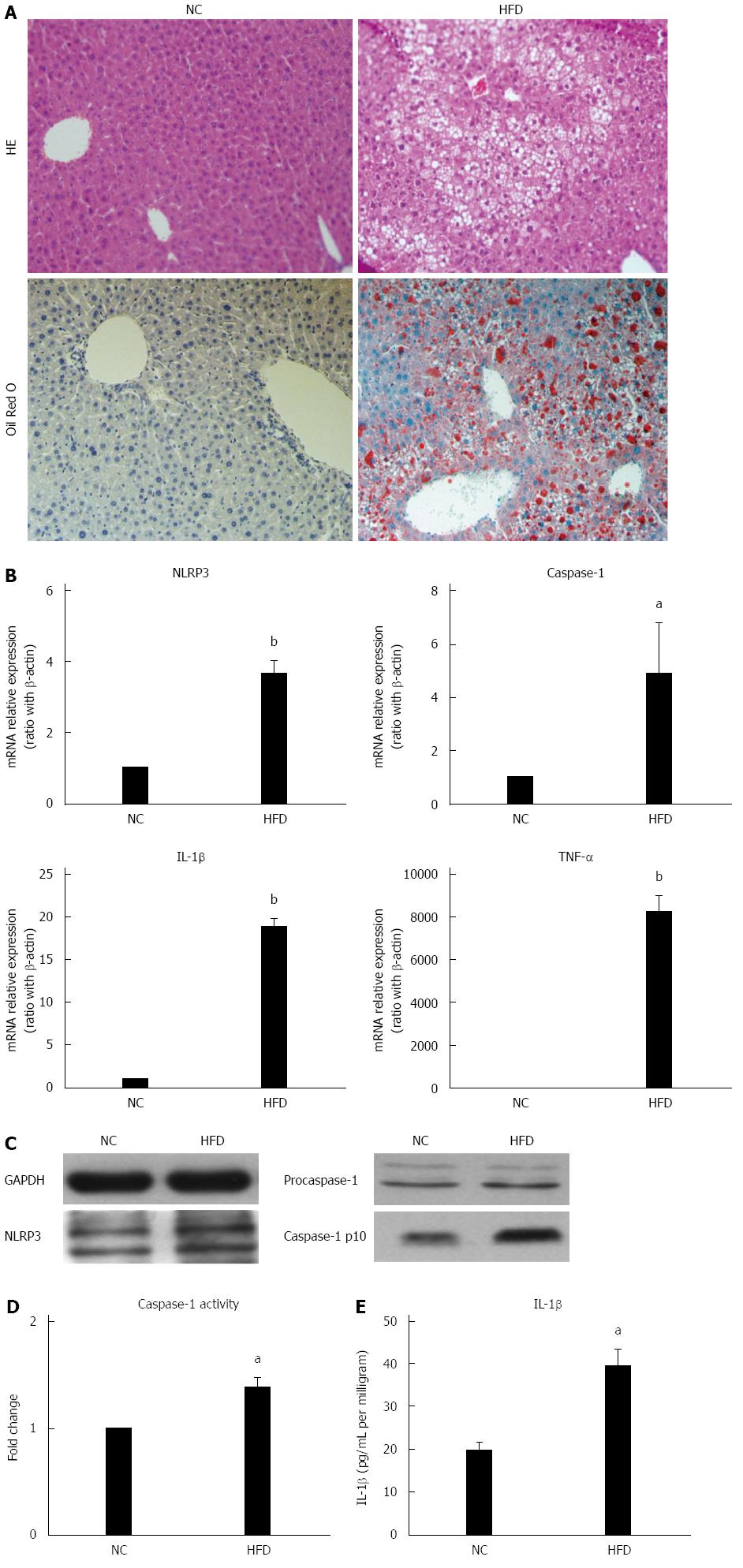
Chronic and systemic activation of the inflammatory-immune system plays an important role in the development of NAFLD[1,2]. Inflammasomes are major contributors to inflammation. Here, the association between high-fat diet feeding and hepatic NLRP3 inflammasome expression was studied. As in our previous report, 14 wk of HFD induced significant but simple hepatic steatosis (Figure 1A) and up-regulated mRNA expression of proinflammatory cytokines TNF-α and IL-1β in liver (Figure 1B) when compared to those of NCD mice. Unlike other proinflammatory cytokines, IL-1β is cleaved from pro-IL-1β by caspase-1, which is activated by the inflammasome complex. Here we found expression of NLRP3 and caspase-1 was significantly up-regulated at mRNA level in the livers of HFD-fed mice versus NCD-fed mice (Figure 1B). Furthermore, the protein expression of NLRP3 and fountional cleavage caspase-1 (caspase-1-p10) was also increased in HFD-fed mice (Figure 1C). In agreement with the increased fountional caspase-1 mRNA expression, caspase-1 activity (Figure 1D) and mature IL-1β protein levels (Figure 1E) in liver tissue were significantly increased in HFD-fed mice versus NC-fed mice, which indicated inflammasome activation. Taken together, these results indicated that high-fat diet induced hepatic NLRP3 inflammasome activation is associated with hepatic steatosis.
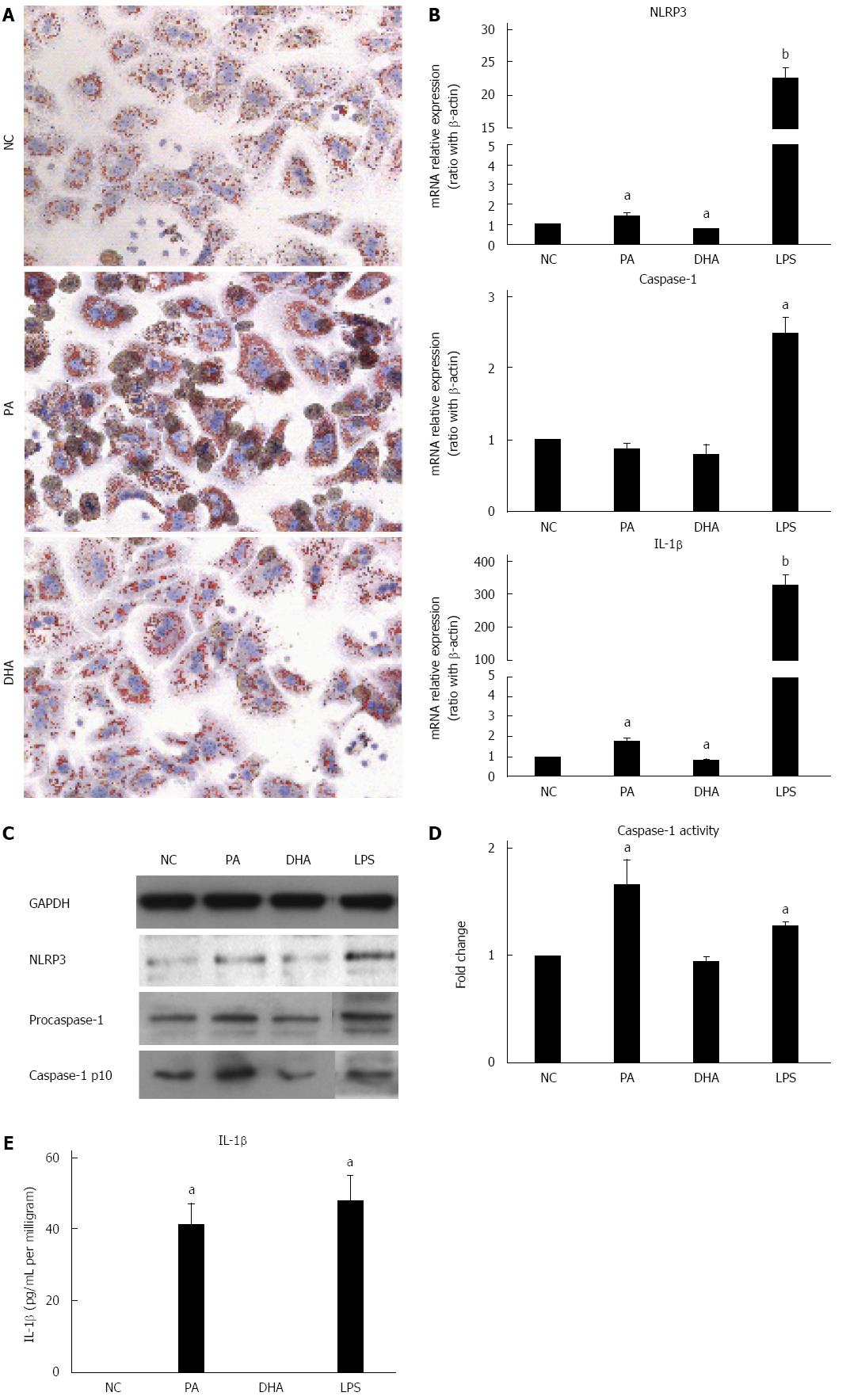
Hepatic steatosis was characterized with overloading of lipid droplets in hepatocytes. Next, whether lipid droplets deposition in hepatocytes can directly induce and activate NLRP3 inflammasome was investigated and the effect of different fatty acids on NLRP3 inflammasome activation was explored further. Primary hepatocytes isolated from C57BL/6 mice were incubated in either SFA (PA, 0.5 mmol/L) or PUFA (DHA, 50 μmol/L) for 24 h. Oil red O staining showed severe lipid droplet deposition in PA-treated hepatocytes when compared to those of normal controls. However, slight lipid droplet deposition was observed in DHA-treated hepatocytes (Figure 2A), which was similar to that of normal controls. As expected, PA-treated hepatocytes demonstrated markedly increased mRNA expression of NLRP3 and IL-1β compared to normal controls (Figure 2B). The protein expression of NLRP3 and fountional caspase1-p10 in hepatocytes after PA treatment was also increased (Figure 2C). Moreover, caspase-1 activity (Figure 2D) and IL-1β secretion (Figure 2E) were markedly increased in PA-treated hepatocyte culture supernatants. In contrast, DHA treatment significantly decreased mRNA expression of NLRP3 and IL-1β compared to normal controls (Figure 2B) but no significant change was detected in caspase-1 activity and IL-1β secretion in culture supernatants. Altogether, these results indicated that SFAs alone could induce and activate NLRP3 inflammasome in hepatocytes, which may be associated with lipid deposition. On the other hand, PUFAs had the potential to inhibit NLRP3 inflammasome activation.
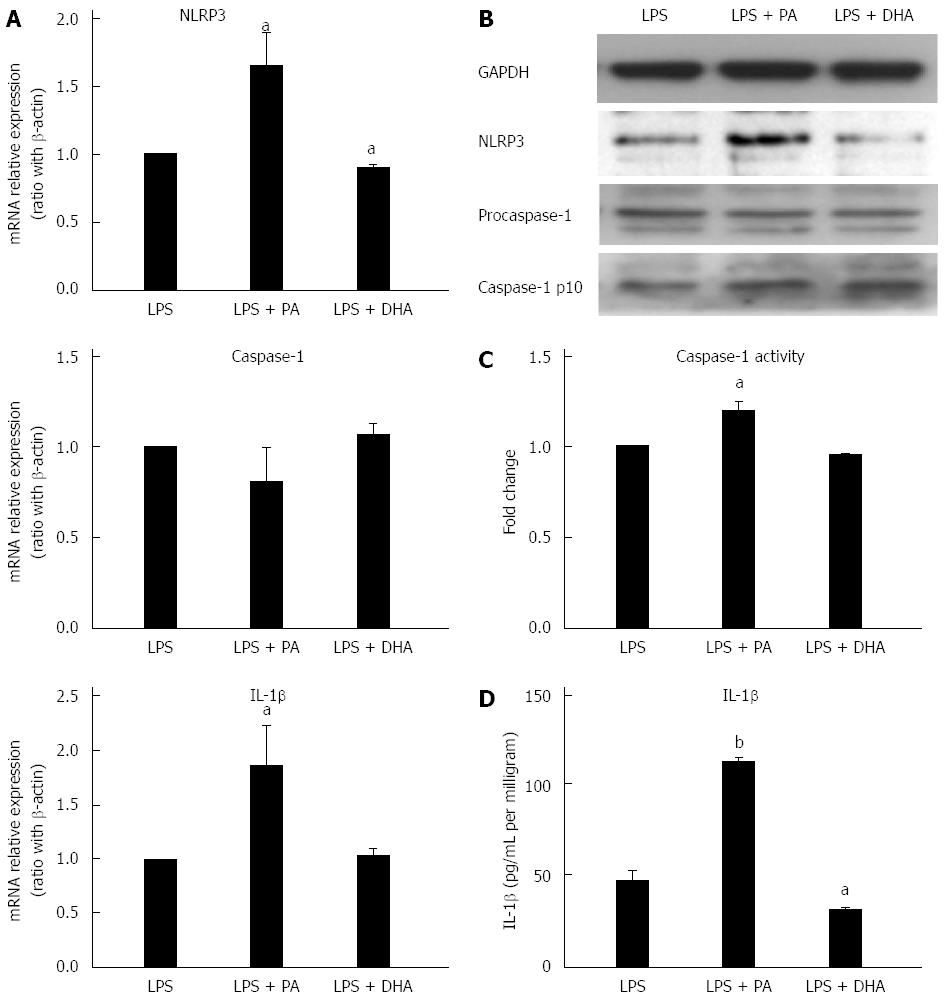
LPS has been proposed as an important factor in the pathogenesis of NAFLD. Recent reports have shown LPS can induce and activate NLRP3 inflammasome in the liver[21]. Furthermore, TLRs and PA cooperatively activated inflammasome in the liver[14,22]. Next, the effect of LPS combined with different fatty acids on NLRP3 inflammasome activation in hepatocytes was investigated. Figure 3A and B shows that PA combined with LPS significantly increased NLRP3 gene and protein expression when compared with LPS treatment alone. Simultaneously, caspase-1 activity (Figure 3C) and IL-1β secretion (Figure 3D) were also significantly increased in the combined treatment group versus LPS treatment alone. In contrast, DHA combined with LPS significantly decreased NLRP3 expression at gene and protein levels when compared with LPS treatment alone (Figure 3A and B). Furthermore, IL-1β secretion in culture supernatant was decreased markedly after DHA combined with LPS treatment (Figure 3C). These results indicated that SFAs enhanced sensitization to LPS-induced NLRP3 inflammasome activation, while PUFAs had the potential to decrease LPS-induced inflammasome activation.
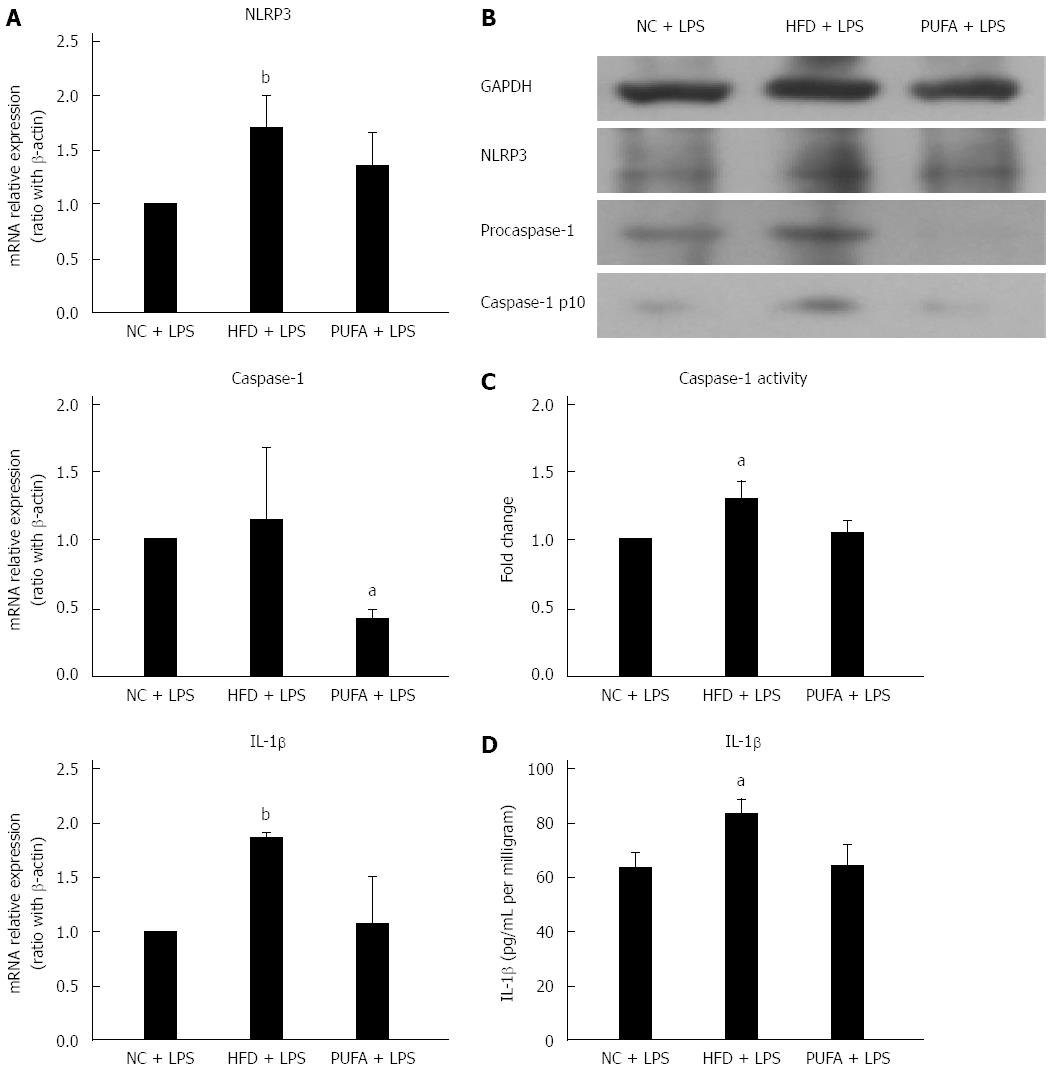
Different fatty acids oppositely regulated LPS-induced NLRP3 inflammasome activation in vitro. Next, whether different fatty acids-enriched diets had different effect on LPS-induced NLRP3 inflammasome activation in vivo was explored. Hepatic NLRP3 inflammasome expression was determined after administration of LPS and 4 wk feeding of either HFD (enriched in SFAs) or high-PUFA diet (enriched in PUFAs). Short-term feeding of HFD significantly enhanced LPS-induced up-regulation of NLRP3 and IL-1β gene expression when compared to NCD (Figure 4A). NLRP3 and caspase-1 protein expression were also up-regulated in mice fed with HFD combined with LPS treatment (Figure 4B). Furthermore, caspase-1 activity and IL-1β production in liver tissue were significantly increased in HFD mice (Figure 4C and D). In contrast, a PUFA-enriched diet significantly decreased LPS-induced caspase-1 gene and protein expression (Figure 4A and B). Altogether, these results revealed that different fatty acids-enriched diet offered opposite sensitization to LPS-induced NLRP3 inflammasome activation.
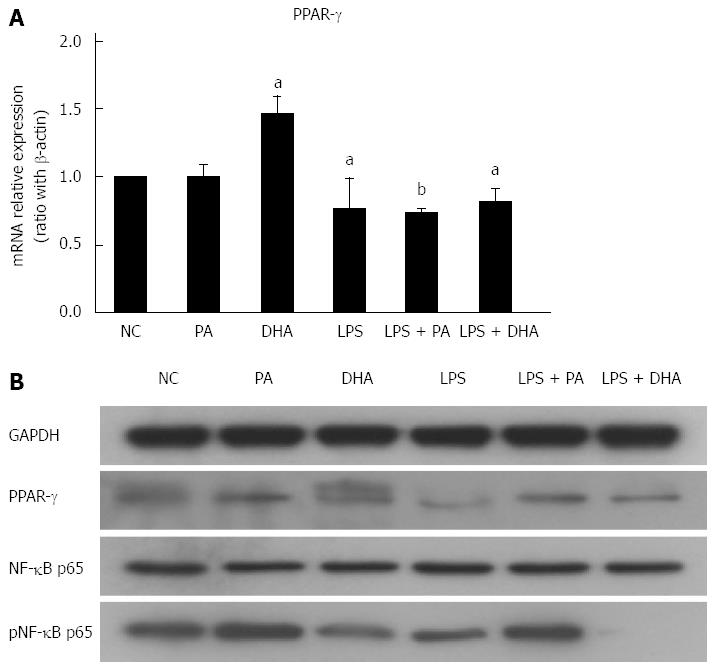
Recent study has shown that NF-κB plays a critical role in priming NLRP3 inflammasome. Furthermore, PPAR-γ inhibited proinflammatory cytokine expression partly by antagonizing activities of the transcription factors NF-κB. In the current study, we determined NF-κB/PPAR-γ expression in hepatocytes treated with different fatty acids. Figure 5A shows that DHA treatment significantly increased PPAR-γ gene expression in hepatocytes, but LPS alone as well as combined with either PA or DHA treatment decreased PPAR-γ expression. However, PPAR-γ protein expression was similar in PA and DHA-treated hepatocytes (Figure 5B). On the other hand, we found PA increased but DHA decreased NF-κB p65 protein expression in hepatocytes. Furthermore, PA combined with LPS had a synthetic effect on p65 NF-κB protein expression, while DHA almost completely abolished LPS-induced NF-κB activation. Therefore, different fatty acids exerted opposite effects on the inflammatory response partly through regulating the NF-κB activity.
Increasing evidence has suggested that inflammasome plays an important role in the insulin resistance and development of NAFLD[7-9]. In the current study, we confirmed that HFD-induced hepatic simple steatosis was sufficient to induce and activate hepatic NLRP3 inflammasome. Furthermore, different fatty acids had opposite effects on the NLRP3 inflammasome in hepatocytes. SFAs significantly activated the NLRP3 inflammasome, while n-3 PUFAs had the potential to decrease the activity of NLRP3 inflammasome. In addition, different fatty acids transmitted different sensitization to LPS-induced activation of NLRP3 inflammasome in vitro and in vivo. The different effects of fatty acids on NLRP3 inflammasome may be dependent on the NF-κB/PPAR-γ signaling.
Previous study has shown up-regulation and activation of NLRP3 inflammasome in mice models of long-term (9 mo) HFD-induced NASH, while there was no inflammasome activation in mice models of short-term (4 wk) HFD[14]. In the current study, 14 wk of HFD feeding induced significant hepatic steatosis and increased mRNA expression of TNF-α and IL-1β. Furthermore, hepatic expression of NLRP3 and caspase-1 were up-regulated at both mRNA and protein levels and caspase-1 activity and IL-1β secretion in the liver of HFD-fed mice were significantly increased, which indicated inflammasome activation. Our previous studies showed no significant lipid accumulation in the liver of short-term (4-6 wk) HFD-fed mice, but 8-12 wk feeding of HFD induced markedly hepatic steatosis[23]. Therefore, HFD-induced hepatic steatosis was a prerequisite to induce and activate NLRP3 inflammasome. It has been widely acknowledged that NLRP3 inflammasome is activated by PAMPs or DAMPs[5,6,24]. Excessive intracellular lipid droplet deposition has been thought as one of the endogenous DAMPs[12]. Therefore, hepatic steatosis may initiate NLRP3 inflammasome gene expression through metabolic stress-induced endogenous DAMPs. Furthermore, FFAs derived from HFD could directly stimulate TLRs and activate inflammasome as a second stimulus. Altogether, HFD-induced hepatic steatosis was sufficient to induce and activate hepatic NLRP3 inflammasome, which may contribute to the development of NAFLD.
FFAs, usually elevated in the plasma of NASH patients and animal models of NAFLD, have been proposed to promote inflammatory responses by directly engaging TLRs and inducing NF-κB-dependent production of inflammatory cytokines[10-12]. Recently, emerging evidence demonstrated that FFAs activated the inflammasome[13,14,25]. Wen et al[13] demonstrated the SFA palmitate could activate the NLRP3 inflammasome-dependent IL-1β secretion from murine macrophages. Reynolds CM found palmitate induced IL-1β secretion in dendritic cells through a caspase-1/ASC/NLRP3-dependent pathway[25]. These results implicated that dietary SFAs served as DAMPs to induce NLRP3 inflammasome; however, SFAs only showed a priming role on NLRP3 inflammasome. In the current study, we found exposure to dietary PA induced significant lipid deposition in hepatocytes. The lipid-loaded hepatocytes exhibited not only up-regulated NLRP3 inflammasome expression and pro-IL-1β gene transcription, but also increased caspase-1 activity and IL-1β secretion. Our results indicated that SFAs alone could directly induce and activate NLRP3 inflammasome and initiate inflammatory responses in hepatocytes. The discrepancy between ours and others was mainly due to the different cell types studied. Hepatocytes were the major place of lipid metabolism and excessive lipid deposition in hepatocytes led to lipotoxicity. Recent study has demonstrated that SFAs enhanced endoplasmic reticulum stress and induced production of ROS, which were sufficient for the induction of IL-1β production via activation of the NF-κB and inflammasome pathways[26]. Therefore, endogenous DAMPs associated with excessive intracellular lipid deposition may serve as both a priming and activating signal on NLRP3 inflammasome. On the other hand, we found n-3 PUFAs had no effect on IL-1β secretion and it even decreased NLRP3 inflammasome gene expression to some extent. Recently, Yan et al[16] reported that omega-3 fatty acids inhibited the NLRP3 inflammasome activation in macrophages. L’homme et al[15] found unsaturated fatty acids abolished SFA-induced NLRP3 inflammasome activation and inhibited subsequent caspase-1 activation and IL-1β secretion in human monocytes/macrophages. These results indicated different dietary fatty acids exerted opposite roles in the activation of the NLRP3 inflammasome. Taken together, SFAs, the main fatty acids derived from HFD, may contribute to the development of NAFLD through activating NLRP3 inflammasome, at least partly. In contrast, PUFAs had the potential to play a protective role in NAFLD.
The level of bacterial endotoxin (or LPS) is increased in the portal and/or systemic circulation in NFALD[27]. Emerging evidence supported the role of gut-derived endotoxin in the pathogenesis of NAFLD. LPS was recognized by TLRs and activated the signaling pathway to produce proinflammatory cytokines such as TNF-α, which further increased lipid accumulation and decreased β-oxidation of fatty acids[28,29]. Previous studies demonstrated LPS directly induced and activated the NLRP3 inflammasome in the liver[21,30]. Furthermore, SFAs enhanced sensitization to LPS-induced inflammasome activation and IL-1β release in hepatocytes. In the current study, we found that SFAs increased but PUFAs decreased sensitization to LPS-induced NLRP3 inflammasome activation and subsequent IL-1β secretion in hepatocytes. A similar result was observed in vivo. HFD increased sensitization to LPS-induced hepatic NLRP3 inflammasome activation in vivo, while a PUFA-enriched diet had the potential to inhibit LPS-induced hepatic NLRP3 inflammasome activation to some extent. Taken altogether, HFD or SFAs and LPS cooperatively contributed to NLRP3 inflammasome activation, which may play an important role in the progression of NAFLD to NASH. In contrast, PUFAs had an inhibitory effect on NLRP3 inflammasome and decreased LPS-induced NLRP3 inflammasome activation, indicating a new protective and anti-inflammatory function of PUFAs.
We found different fatty acids or diets oppositely regulated the activity of NLRP3 inflammasome, but the underlying possible mechanism involved was not elucidated. It has been shown that SFAs were able to activate TLR4 signaling to induce gene expression of diverse proinflammatory cytokines by regulating the transcription factors, including NF-κB[12]. In contrast, PUFAs were verified to reduce lipid accumulation and improve insulin sensitivity through regulating transcription factors, such as PPAR-γ, to exert anti-inflammatory responses[31]. Recently, studies have shown that NF-κB signaling was necessary for proper activation of the NLRP3 inflammasome. Bauernfeind FG demonstrated that NF-κB inhibition led to reduction of NLRP3 inflammasome expression induced by LPS[30]. Furthermore, some studies demonstrated that PPAR-γ inhibited proinflammatory cytokine gene expression partly by antagonizing activities of the transcription factors AP-1, STAT and NF-κB[32]. In the present study, we found SFAs increased but PUFAs decreased NF-κB p65 protein expression in hepatocytes. Furthermore, SFAs combined with LPS had a synthetic effect on p65 NF-κB protein expression, while PUFAs almost completely abolished LPS-induced NF-κB activation. However, PPAR-γ protein expression was similar in hepatocytes treated with SFAs or PUFAs. Our result was similar to other reports. Recently, Williams-Bey Y found that DHA suppressed macrophage inflammasome activation by inhibiting NF-κB activation and also potently reduced LPS-induced nuclear translocation of p65 NF-κB[33]. Therefore, different dietary fatty acids oppositely regulated the activity of NLRP3 inflammasome through directly regulating NF-κB activation, which may play a crucial role in the progression or improvement of NAFLD.
In conclusion, our study confirmed that HFD-induced hepatic steatosis was sufficient to induce and activate hepatic NLRP3 inflammasome, which played an important role in the development of NAFLD. Moreover, SFAs directly activated NLRP3 inflammasome and increased sensitization to LPS-induced inflammasome activation, while PUFAs had the potential to inhibit NLRP3 inflammasome expression and abolished LPS-induced NLRP3 inflammasome activation. Different fatty acids oppositely regulated NLRP3 inflammasome activity through direct activation or inhibition of NF-κB.
Increasing evidence has suggested chronic and systemic low-grade inflammation plays an important role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Free fatty acids have been proposed as a major contributing factor for inflammation. Inflammasome has emerged as an important regulator of inflammation.
Recent studies suggest a critical role for the NOD-like receptor protein 3 (NLRP3) inflammasome in obesity and its complications. Elevated reactive oxygen species levels, mitochondria damage and lysosomal rupture have been proposed to participate in NLRP3 inflammasome activation but little is known about fatty acids to positively or negatively regulate NLRP3 inflammasome activation.
This research inventively demonstrated that hepatic NLRP3 inflammasome activation played an important role in the development of NAFLD. Dietary saturated fatty acids (SFAs) and polyunsaturated fatty acids (PUFAs) oppositely regulated the activity of NLRP3 inflammasome through direct activation or inhibition of nuclear factor-kappa B (NF-κB).
This research confirmed that dietary SFAs and PUFAs oppositely affect hepatic NLRP3 inflammasome through regulating NF-κB activation, which may provide a new therapy option of a dietary-based intervention for NAFLD.
The NLRP3 inflammasome is a cytosolic protein complex composed of the regulatory subunit NLRP3, the adaptor apoptosis-associated speck-like protein, and the effector caspase-1. Two-signal hypothesis is accepted in inflammasome activation: the first signal produces and induces pro-Interleukine-1 beta (pro-IL-1β) and inflammasome components expression; and the second signal assembles inflammasome components to engage in caspase-1 activation and secret bioactive IL-1β.
The authors investigated the effect of different dietary fatty acids on hepatic inflammasome activation. The work was logically designed and nicely described.
P- Reviewer: Kucera O, Lee MK S- Editor: Gong ZM L- Editor: Roemmele A E- Editor: Ma S
| 1. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5683] [Cited by in RCA: 6285] [Article Influence: 349.2] [Reference Citation Analysis (1)] |
| 2. | Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1764] [Article Influence: 135.7] [Reference Citation Analysis (1)] |
| 4. | Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 5. | Martinon F. Detection of immune danger signals by NALP3. J Leukoc Biol. 2008;83:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 549] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 7. | Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1698] [Cited by in RCA: 2005] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 8. | Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:15324-15329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 570] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 9. | Dixon LJ, Flask CA, Papouchado BG, Feldstein AE, Nagy LE. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One. 2013;8:e56100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 513] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 12. | Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2724] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 13. | Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1393] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 14. | Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 515] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 15. | L’homme L, Esser N, Riva L, Scheen A, Paquot N, Piette J, Legrand-Poels S. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. J Lipid Res. 2013;54:2998-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 565] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 17. | Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243:136-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 633] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 18. | Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1681] [Cited by in RCA: 2058] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 19. | Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3253] [Cited by in RCA: 4154] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 20. | Hua J, Ma X, Webb T, Potter JJ, Oelke M, Li Z. Dietary fatty acids modulate antigen presentation to hepatic NKT cells in nonalcoholic fatty liver disease. J Lipid Res. 2010;51:1696-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Ganz M, Csak T, Nath B, Szabo G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J Gastroenterol. 2011;17:4772-4778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 23. | Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3880] [Cited by in RCA: 4482] [Article Influence: 298.8] [Reference Citation Analysis (0)] |
| 25. | Reynolds CM, McGillicuddy FC, Harford KA, Finucane OM, Mills KH, Roche HM. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol Nutr Food Res. 2012;56:1212-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Kim S, Joe Y, Jeong SO, Zheng M, Back SH, Park SW, Ryter SW, Chung HT. Endoplasmic reticulum stress is sufficient for the induction of IL-1β production via activation of the NF-κB and inflammasome pathways. Innate Immun. 2014;20:799-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond). 2010;7:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1727] [Cited by in RCA: 2286] [Article Influence: 142.9] [Reference Citation Analysis (1)] |
| 31. | Depner CM, Traber MG, Bobe G, Kensicki E, Bohren KM, Milne G, Jump DB. A metabolomic analysis of omega-3 fatty acid-mediated attenuation of western diet-induced nonalcoholic steatohepatitis in LDLR-/- mice. PLoS One. 2013;8:e83756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2807] [Cited by in RCA: 2801] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 33. | Williams-Bey Y, Boularan C, Vural A, Huang NN, Hwang IY, Shan-Shi C, Kehrl JH. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS One. 2014;9:e97957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |