Published online Feb 14, 2016. doi: 10.3748/wjg.v22.i6.2092
Peer-review started: August 3, 2015
First decision: September 9, 2015
Revised: September 27, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: February 14, 2016
AIM: To evaluate the therapeutic effects of bone marrow-derived mesenchymal stem cells (BMSCs) with human urokinase-type plasminogen activator (uPA) on liver fibrosis, and to investigate the mechanism of gene therapy.
METHODS: BMSCs transfected with adenovirus-mediated human urokinase plasminogen activator (Ad-uPA) were transplanted into rats with CCl4-induced liver fibrosis. All rats were sacrificed after 8 wk, and their serum and liver tissue were collected for biochemical, histopathologic, and molecular analyzes. The degree of liver fibrosis was assessed by hematoxylin and eosin or Masson’s staining. Western blot and quantitative reverse transcription-polymerase chain reaction were used to determine protein and mRNA expression levels.
RESULTS: Serum levels of alanine aminotransferase, aminotransferase, total bilirubin, hyaluronic acid, laminin, and procollagen type III were markedly decreased, whereas the levels of serum albumin were increased by uPA gene modified BMSCs treatment. Histopathology revealed that chronic CCl4-treatment resulted in significant fibrosis while uPA gene modified BMSCs treatment significantly reversed fibrosis. By quantitatively analysing the fibrosis area of liver tissue using Masson staining in different groups of animals, we found that model animals with CCl4-induced liver fibrosis had the largest fibrotic area (16.69% ± 1.30%), while fibrotic area was significantly decreased by BMSCs treatment (12.38% ± 2.27%) and was further reduced by uPA-BMSCs treatment (8.31% ± 1.21%). Both protein and mRNA expression of β-catenin, Wnt4 and Wnt5a was down-regulated in liver tissues following uPA gene modified BMSCs treatment when compared with the model animals.
CONCLUSION: Transplantation of uPA gene modified BMSCs suppressed liver fibrosis and ameliorated liver function and may be a new approach to treating liver fibrosis. Furthermore, treatment with uPA gene modified BMSCs also resulted in a decrease in expression of molecules of the Wnt signaling pathway.
Core tip: It has been confirmed that urokinase plasminogen activator (uPA) has a protective effect in liver fibrosis. Bone marrow-derived mesenchymal stem cells (BMSCs) have been discovered to provide effective therapy for liver fibrosis. Therefore, the present study was designed to investigate the therapeutic effects of uPA gene modified BMSCs in a rat model of CCl4-induced liver fibrosis, and the impact on the Wnt signaling pathway which is involved in the pathogenesis of liver fibrosis. uPA gene modified BMSCs can suppress liver fibrosis and ameliorate liver function. Furthermore, it also resulted in down-regulation of molecules of the Wnt signaling pathway and may be a new approach to treating liver fibrosis.
- Citation: Ma ZG, Lv XD, Zhan LL, Chen L, Zou QY, Xiang JQ, Qin JL, Zhang WW, Zeng ZJ, Jin H, Jiang HX, Lv XP. Human urokinase-type plasminogen activator gene-modified bone marrow-derived mesenchymal stem cells attenuate liver fibrosis in rats by down-regulating the Wnt signaling pathway. World J Gastroenterol 2016; 22(6): 2092-2103
- URL: https://www.wjgnet.com/1007-9327/full/v22/i6/2092.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i6.2092
Liver fibrosis results from a sustained wound-healing response of the liver to a chronic injury, including viral infection, alcohol abuse, drug toxicity and autoimmune hepatopathy. It is characterized by excessive remodeling of extracellular matrix (ECM) and deposition of collagen[1]. The activation of quiescent hepatic stellate cells (HSCs) to a myofibroblast-like phenotype is considered the key event in the pathogenesis of liver fibrosis[2]. Currently, aside from liver transplantation, no other effective strategy exists to reverse or prevent fibrosis. Therefore, other approaches to treating fibrosis need to be investigated.
Bone marrow-derived mesenchymal stem cells (BMSCs) are non-hematopoietic cells with multi-lineage potential[3,4]. They can be isolated from bone marrow and various other sources. BMSCs were recently discovered to provide effective therapy for liver fibrosis because of their multipotent differentiation, high self-renewal ability, and low immunogenicity[5,6]. Several studies have demonstrated that BMSCs could reduce liver fibrosis or improve the liver function in rats with carbon tetrachloride (CCl4)-induced liver fibrosis[7,8]. However, the therapeutic effects were limited and needed to be improved[9]. BMSCs have a particular pattern of cell proliferation and differentiation, enabling easy introduction and expression of the foreign gene and making it a potentially targeted cell for genetic treatment. Therefore, gene modified BMSCs may be a novel therapy to treat liver fibrosis.
For the continuing progress in the field of gene therapy for liver fibrosis, intensive efforts have been put in to design gene therapy strategies. Furthermore, the focus is on blocking any of the fibrogenic pathways and regulating the fibrinolytic homeostasis in a liver fibrosis animal model[1]. The plasminogen activation system is involved in proteolysis, cell migration, tissue remodeling, and cell adhesion. Amongst them the urokinase plasminogen activator (uPA) system has been recently implicated in the inhibition of liver fibrosis[10,11]. The uPA system consists of the serine protease uPA, its inhibitors, and receptor[1]. uPA is a particular serine protease that converts inactive plasminogen into active plasmin, which degrades ECM directly and catalyzes the activation of latent matrix metalloproteinases (MMPs)[12]. Studies of knockout mice with an inactive uPA system have showed that uPA was involved in the pathological liver process after injury and the knockout mice had abnormally high liver fibrogenesis[13]. The role of uPA has also been investigated in rats using CCl4. In the CCl4-induced acute liver injury model, lack of uPA led to the accumulation of fibrin and fibronectin within injured areas, insufficient removal of necrotic cells, and delayed repair. Similarly, plasminogen deficiency also caused excessive matrix accumulation and prominent activation of HSCs after liver injury[14]. Moreover, Pohl et al[15] found that there was decreased expression of uPA in cultured-activated HSC, which resulted in low uPA activity and failed to resolve the fibrotic scarring.
Given the evidence above, we believed that uPA is a protective factor in liver fibrosis, therefore, increasing uPA expression in fibrotic liver tissues may reverse fibrosis and regenerate functional hepatocytes. Increasing studies re searching for the effect of uPA gene therapy have been carried out. However, the specific molecular mechanism remains unclear.
The Wnt signaling pathway consists of a highly conserved family of secreted glycoproteins that play an essential role in diverse arrays of biologic processes such as organogenesis, tissue homeostasis, and pathogenesis of many human diseases[16-18]. On the basis of previous studies, the Wnt signaling pathway has been divided into canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) signaling pathways[19,20]. In the canonical Wnt signaling pathway, β-catenin is a chief downstream effector that mediates the Wnt signaling from the cell membrane to the cytoplasm[21]. The non-canonical pathway is characterized by β-catenin independence, including the non-canonical Wnt/Ca2+ pathway and planar cell polarity (PCP) pathway[22].
Lately, accumulating studies show that the Wnt signaling pathway is involved in the pathogenesis of liver fibrosis[16,23,24]. It was reported that the Wnt signaling pathway obviously participates in HSCs activation, leading to liver fibrosis. Cheng et al[16] found that expression of both canonical (β-catenin) and non-canonical (Wnt4 and Wnt5a) Wnt genes was increased approximately 3-12 fold in culture-activated HSCs compared with quiescent HSCs. Kordes et al[25] also showed that the canonical Wnt signaling was active in freshly isolated HSCs from rats. A subsequent analysis of Kyoto encyclopedia of genes and genomes (KEGG) pathway also revealed that Wnt5a was involved in the activation of HSCs and played a role in liver fibrogenesis[26]. Therefore, the Wnt signaling pathway contributes to HSCs activation, leading to excessive ECM deposition. There seems to be some sort of association between the uPA gene and Wnt signaling pathway. To date, no studies have been carried out to investigate the association between uPA gene and Wnt signaling pathway in liver fibrosis.
Hence, in the present study, we introduced the human uPA gene into BMSCs by using an adenoviral vector and investigated its effect on liver fibrosis. The aims of our study were to evaluate the antifibrogenic effect of uPA gene modified BMSCs on liver fibrosis and to investigate the impact of uPA gene modified BMSCs treatment on Wnt signaling pathway.
Male Sprague-Dawley (SD) rats were included in our study (Experimental Animal Center of Guangxi Medical University, China). All the rats were provided with standard feed and water ad libitum and individually housed at a constant temperature (18 °C-20 °C) and humidity (60%-70%) with a 12 h light/dark cycle. All animal experiments were approved by the Institutional Animal Care and Use Committee of Guangxi Medical University and the animal protocol was designed to minimize the pain and discomfort of the animals.
BMSCs derived from the SD rat were purchased from Sciencell Research Laboratories (San Diego, California, United States). Briefly, the cells were seeded at a density of 3 × 103 cells/cm2 in T-25 culture flasks and incubated in Dulbecco’s modified Eagle’s medium-low glucose (DMEM; Gibco, Grand Island, United States) supplemented with 13% fetal bovine serum (FBS; Gibco), 50 U/mL penicillin and 50 mg/mL streptomycin at 37 °C in 50 mL/L carbon dioxide (CO2). The culture medium was replaced after the first 24 h and every three days after that. After primary cultivation, the adherent cells reached almost 80% confluence. The plastic adherent cells were lifted up with 0.25% trypsin, suspended in fresh medium and transferred to a new flask for expansion. BMSCs at passage 4 (P4) were used for subsequent transduction and transplantation experiments.
Replication-deficient E1 and E3 adenoviral vectors coding for non-secreted human uPA (Ad-uPA) cDNA were purchased from Biowit Biotechnologies (Shenzhen, China). The Ad-uPA expressed both green fluorescence protein (GFP) and human uPA. Adenoviral vector without the therapeutic gene (Ad-GFP) was obtained as the control adenovirus. For adenoviral transduction, the P4 BMSCs (1 × 106 cells/well) were seeded into 6-well plates for 24 h. BMSCs were then infected with Ad-uPA at different levels of multiplicity of infection (MOI) (20, 40, 60, 80, and 100). BMSCs were transfected with Ad-GFP under the same condition. After 48-72 h, light and fluorescent microscopy was performed to observe transfection efficiency and cell viability according to GFP expression and cell morphology. The optimal MOI was chosen for both highest GFP expression and viability and used throughout the study.
After the third day of in vitro adenovirus infection, transfected (Ad-uPA or Ad-GFP) and untransfected BMSCs were harvested and solubilized in protein lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing protease inhibitors. Protein concentration was determined with the BCA (bicinchoninic acid) protein assay kit (Beyotime, Jiangsu, China), and protein lysates were aliquoted and stored at -80 °C until use. Equal amounts of protein were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Beyotime, Jiangsu, China) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Boston, United States). The membrane was blocked with 50 g/L skim milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) at room temperature for 2-3 h. After a brief rinse, the membrane was incubated overnight at 4 °C in TBST with a rabbit anti-human uPA antibody (1:6000; Abcam, Cambridge, United States) and horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibodies (1:1000; Cell Signaling, Boston, United States) for 1-2 h at room temperature. Then the membrane was washed in TBST and protein was detected by enhanced chemical luminescence (ECL) (Beyotime Institute of Biotechnology, Haimen, China).
To induce liver fibrosis, 6-8-wk-old male SD rats (m = 200-250 g, n = 30) were injected with 400 mL/L CCl4 subcutaneously (CCl4:olive oil, 2:3) at a dose of 3 mL/kg every three days for 8 wk[27]. Mock-treated rats were injected with olive oil alone as a normal control group (n = 10). At the end of the 4th week, the model rats were randomly divided into three groups: (1) uPA-BMSCs group (injected via the tail vein with 2 × 106 Ad-uPA-transfected BMSCs, n = 10); (2) BMSCs group (injected via the tail vein with 2 × 106 untransfected BMSCs, n = 10); and (3) model group (injected via the tail vein with an equal volume of normal saline, n = 10). Meanwhile, rats in the control group were given the same dose of normal saline. All rats were sacrificed at the end of the 8th week, and biological samples (liver tissues and blood samples) were obtained for molecular and histological analyses. Liver tissue was excised for quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot analysis. The remaining tissue was fixed and processed for histological analysis.
Immediately after blood sample collection, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL) and albumin (ALB) levels in serum were measured with an automated analyser (LX20; Beckman Coulter, Fullerton, CA, United States) at the First Affiliated Hospital of Guangxi Medical University. The levels of serum hyaluronic acid (HA), laminin (LN), and procollagen type III (PCIII) were detected by enzyme-linked immunosorbent assay (ELISA).
Liver tissues were fixed in 40 g/L formaldehyde, embedded in paraffin and sectioned at a thickness of 5 μm. Hematoxylin and eosin (HE) staining and Masson’s trichrome staining were used for histological structure analysis and fibrosis area analysis, respectively. Five random views of Masson trichrome-stained sections from each sample (n = 10/group) were captured by a light microscope (Olympus, Tokyo, Japan). The fibrotic area was checked with the Image J 1.44s software (National Institutes of Health, Bethesda, MD, United States)[28]. The percentage of the fibrotic area was calculated by comparing the collagen stained area to the total area.
qRT-PCR was used to assess the mRNA expression of molecules involved in Wnt signaling (β-catenin, Wnt4, and Wnt5a). Total RNA was extracted from liver tissues of each rats using chloroform and Trizol solution (TaKaRa Bio Inc, Shiga, Japan). First-strand cDNA synthesis was performed with 1 μg of total RNA. Moreover, cDNA samples were thereafter amplified in the ABI Prism 7500 Sequence Detection system (Applied Biosystems, Massachusetts, United States) for 40 cycles (95 °C for 3 s, 60 °C for 34 s) with specific oligonucleotide primers (TaKaRa Bio Inc., Shiga, Japan). Each sample was analyzed in triplicate, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used for normalization. The relative quantification of target genes was determined using the ΔΔCT method[29]. Primers used in qRT-PCR analyzes are listed in Table 1.
| Gene name | Direction | Sequence (5’-3’) |
| β-catenin | Forward | ACGGCAATCAGGAAAGCAA |
| Reverse | ACAGACAGCACCTTCAGCACTC | |
| Wnt4 | Forward | GCCATCTCTTCAGCAGGTGTG |
| Reverse | CATAGGCGATGTTGTCCGAGC | |
| Wnt5a | Forward | ACTTGCACAACAATGAAGCAGGTC |
| Reverse | CATAGGCGATGTTGTCCGAGC | |
| uPA | Forward | CGAAGACTTCAGCGACGAAAC |
| Reverse | CGAAGACTTCAGCGACGAAAC | |
| GAPDH | Forward | TATGACTCTACCCACG |
| Reverse | ATACTCAGCACCAGCATCACC |
Total protein from liver tissue samples was extracted by the standard procedure[30]. The BCA assay was used to estimate protein concentration. The following primary antibodies were used: rabbit anti-rat uPA antibody (1:6000 dilution; Abcam, Cambridge, United States), mouse anti-rat β-catenin antibody (1:1000 dilution; Origene Technologies, Maryland, United States), goat anti-rat Wnt4 antibody (1:5000 dilution; Origene Technologies), and rabbit anti-rat Wnt5a antibody (1:1000 dilution; Origene Technologies). Western blot was done using the method described above.
All data are expressed as the mean ± SD. Comparisons between groups were performed using one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test. P-values less than 0.05 were considered statistically significant. All the statistical analyses were performed with the statistical software package SPSS version 16.0 (SPSS Inc., Chicago, United States).
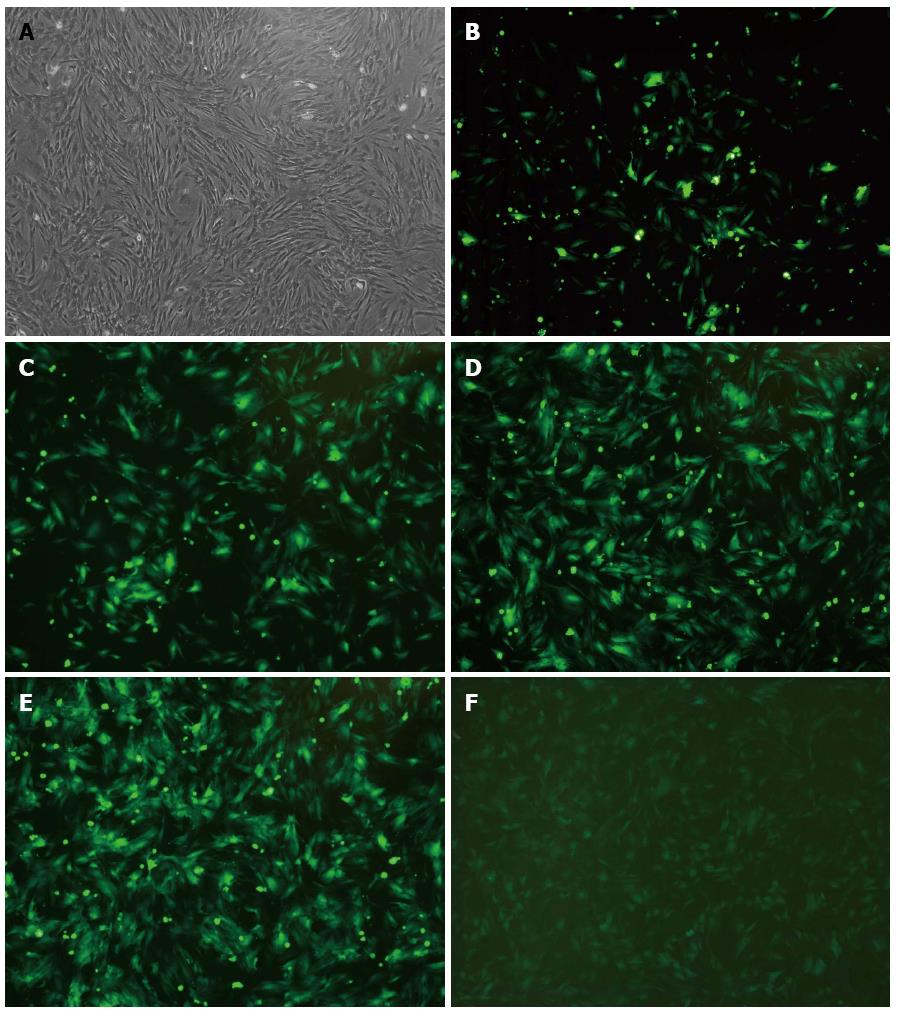
Rat BMSCs were successfully isolated and cultured in whole bone marrow adherent culture system. As shown in Figure 1A, after being subcultured four times, the cells remained in good status and the shape of BMSCs changed from round shape initially to long spindle shape after that. Meanwhile, it was found that the P4 BMSCs formed colonies and distributed radially in the flask.
To test the transfection efficiency, BMSCs were infected with adenoviral vectors (Ad-uPA or Ad-GFP) at MOI of 20, 40, 60, 80 and 100. As shown in Figure 1B-F, 72 h later, BMSCs were found to be in good condition when transfected at an MOI of 80 under the fluorescence microscope, with highest cell viability and transfection efficiency. The BMSCs were in better status when transfected at MOI of 20, 40 and 60. However, it featured poor transfection efficiency. There was strong green fluorescence at an MOI of 100, yet under the microscope, morphology retraction was found in some of the adherent cells as well as a few floating cells. The result suggested that 80 was the optimal MOI to be used for adenoviral infection.
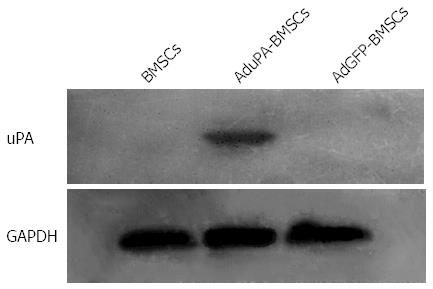
To further determine the expression of uPA in Ad-uPA-transfected BMSCs, Western blot analysis was performed. As showed in Figure 2, there was no positive band in Ad-GFP-transfected BMSCs and un-transfected BMSCs. However, a specific band was only found in Ad-uPA-transfected BMSCs. These results indicated that uPA adenovirus had been successfully transfected into BMSCs, leading to significant expression of uPA in vitro.
As shown in Table 2, rats in the model group displayed chronic liver injury, with higher serum ALT, AST, and TBIL levels and lower ALB levels than in the control group (P < 0.01 for all). However, the levels of ALT, AST, and TBIL were significantly decreased, whereas the levels of ALB were increased in the uPA-BMSCs group compared with the model group (P < 0.05 for all). Serum ALT, AST, and TBIL levels in the BMSCs group were also decreased but higher than those in the uPA-BMSCs group. Moreover, as compared to the control group, higher levels of serum HA, LN, and PCIII were detected in the model group. Moreover, the BMSCs treatment significantly reduced all the above parameters compared with the model group (P < 0.05 for all) with an enhanced effect in the uPA-BMSCs group.
| Group | n | ALT (U/L) | AST (U/L) | TBIL (μmol/L) | ALB (g/L) | HA (μg/L) | LN (μg/L) | PCIII (μg/L) |
| uPA-BMSCs | 10 | 74.95 ± 12.66bc | 173.68 ± 21.10bc | 18.66 ± 2.52bc | 30.63 ± 4.25bc | 105.71 ± 15.21bc | 143.82 ± 18.99bc | 27.14 ± 5.93bc |
| BMSCs | 10 | 96.34 ± 14.97b | 228.65 ± 25.66b | 23.95 ± 5.09b | 26.65 ± 3.79b | 127.24 ± 17.79b | 173.15 ± 22.32b | 53.38 ± 6.31b |
| Model | 10 | 126.76 ± 19.29a | 287.67 ± 26.17a | 32.13 ± 7.11a | 22.60 ± 4.35a | 187.66 ± 22.88a | 224.45 ± 23.40a | 73.89 ± 10.76a |
| Control | 10 | 31.45 ± 7.75 | 67.51 ± 11.86 | 13.39 ± 3.23 | 35.95 ± 5.02 | 47.15 ± 6.58 | 97.23 ± 17.59 | 16.13 ± 3.13 |
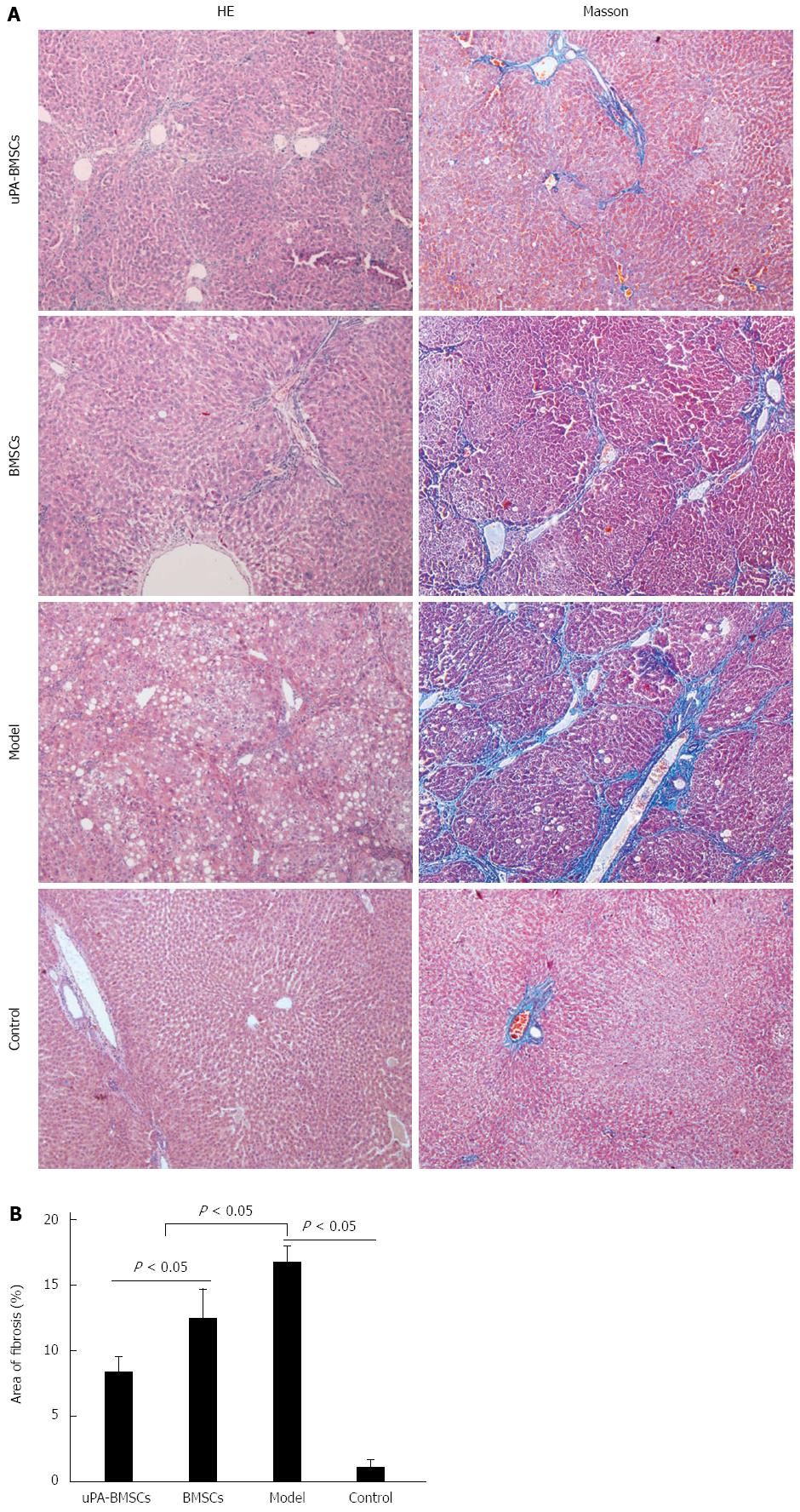
Histological examination using HE and Masson’s staining were performed to show the extent of liver damage (Figure 3A). For HE staining, liver tissue samples from the normal control group showed normal lobular architecture with radiating hepatic cords pointing to central veins, whereas the model group exhibited fatty degeneration, ballooning changes of hepatocytes and necrosis. In contrast, uPA-BMSCs treatment remarkably ameliorated the adipose degeneration of hepatocytes and reduced the immigration of inflammatory cells compared with the model group or BMSCs group. For Masson’s staining, the control group showed the normal architecture while the model group presented extensive liver bridging fibrosis and substantial collagen deposition. However, bridging fibrosis and collagen were distinctly decreased by the uPA-BMSCs or BMSCs treatment compared with the model group (Figure 3A). Also, quantitative analyzes of fibrosis area were consistent with the histological changes. The model group had the largest fibrotic area. Fibrotic area was significantly decreased by BMSCs treatment and was further reduced by uPA-BMSCs treatment (Figure 3B, control group, 1.03% ± 0.66% vs model group, 16.69% ± 1.30% vs BMSCs group, 12.38% ± 2.27% vs uPA-BMSCs group, 8.31% ± 1.21%; control group vs model group, P < 0.05; model group vs BMSCs group, P < 0.05; model group vs uPA-BMSCs group, P < 0.05; BMSCs group vs uPA-BMSCs group, P < 0.05).
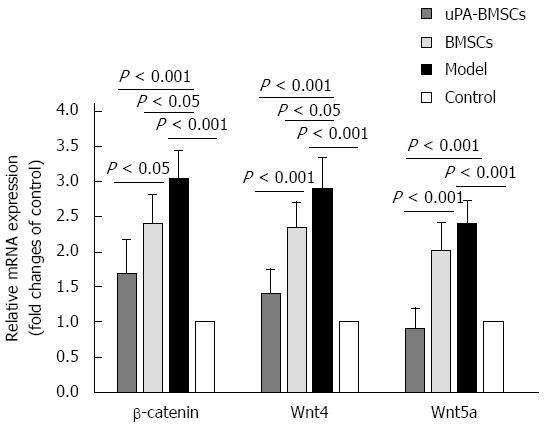
We examined β-catenin, Wnt4, and Wnt5a mRNA expression levels in liver tissues by qRT-PCR (Figure 4). As revealed in Figure 4, low mRNA expression levels of β-catenin, Wnt4, and Wnt5a were detected in the control group, while they were significantly increased in the model group (P < 0.05 for all). Interestingly, uPA-BMSCs treatment could further reduce the expression levels of all these indexes compared with the model group or BMSCs group (P < 0.05 for all). According to these results, we hypothesized that uPA-BMSCs treatment could attenuate liver fibrosis via mechanisms that may be associated with decreased activation of the Wnt signaling pathway.
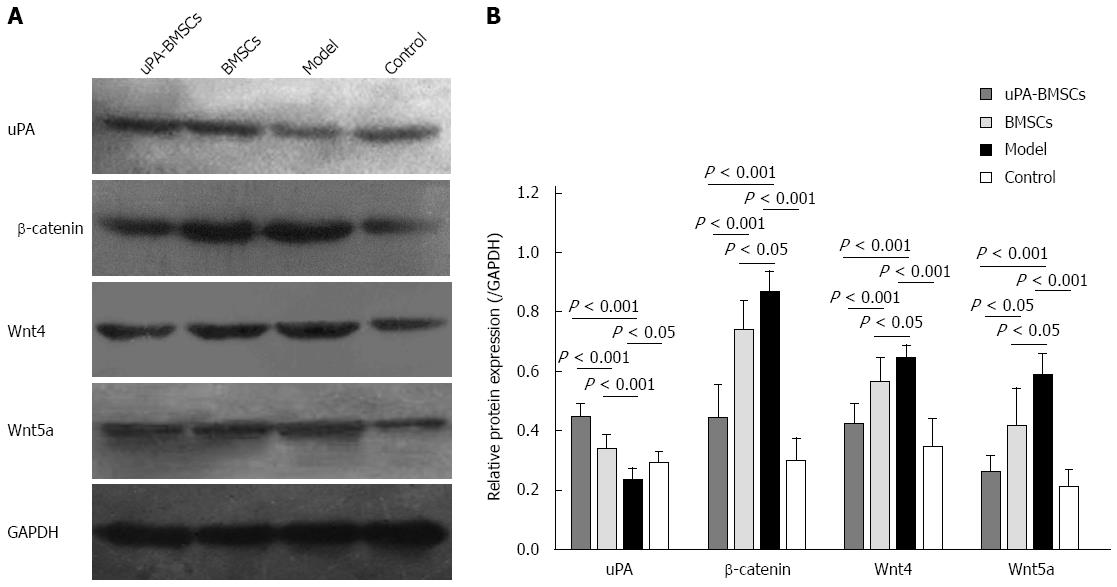
It has been widely accepted that the Wnt signaling pathway participates in liver fibrosis. To further explore the association between the antifibrogenic effect of uPA-BMSCs transplantation and the Wnt signaling pathway in liver fibrosis, we examined the protein expression levels of uPA, β-catenin, Wnt4, and Wnt5a in liver tissues by Western blot (Figure 5A). The densitometric quantification (Figure 5B) of these bands showed that protein expression of uPA was apparently increased in the uPA-BMSCs group, suggesting the successful expression of uPA in vivo. Also, the protein expression levels of β-catenin, Wnt4, and Wnt5a were significantly increased in the model group compared with the control group (P < 0.05 for all). However, the uPA-BMSCs group had markedly lower protein expression levels of β-catenin, Wnt4, and Wnt5a than the model group or BMSCs group (P < 0.05 for all). Moreover, the BMSCs group displayed a slight increase in the expression levels of uPA with a slight decrease of β-catenin, Wnt4, and Wnt5a compared with the model group (P < 0.05 for all).
Liver fibrosis is a reversible consequence of chronic damage to the liver mainly in conjunction with the excessive deposition of ECM proteins in the liver[1]. Progression of liver fibrosis eventually leads to cirrhosis, which can be associated with hepatocellular carcinoma and liver failure. Early intervention or treatment of liver fibrosis greatly reduces the risk of cirrhosis. However, there is no effective drugs to attenuate liver fibrosis presently. Therefore, new therapy strategies are being intensely investigated. In this study, we made a new attempt to deliver uPA gene modified BMSCs into rats with CCl4-induced liver fibrosis.
Numerous previous studies have showed that BMSCs can improve tissue repair, reduce inflammation[31,32], and differentiate into hepatic cells in vitro and in vivo[33,34]. As a result, BMSCs have attracted much attention over the past decade as a novel therapeutic paradigm for chronic liver diseases, such as liver failure[35]. Both Sakaida et al[36] and Fang et al[37] showed that transplantation of BMSCs reduced CCl4-induced liver damage and collagen deposition in mice. A therapeutic effect of transplanting BMSCs was also documented in CCl4-injured rats[7]. In our study, we found that the serum levels of liver function indexes were improved in the BMSCs treatment group as compared with the CCl4-induced liver fibrosis rat model group. Meanwhile, BMSCs treatment significantly attenuated bridging fibrosis. These results were consistent with the previous studies. However, some reports indicated that the therapeutic effect of simple BMSCs therapy has been limited[38-40]. Although BMSCs may enhance the regeneration of hepatocytes, it is hard to break down the reconstructed fibrous scar, even with increased secretion of anti-fibrotic factors. Therefore, the genetic modification of BMSCs might be a more promising option for improving their therapeutic potential.
In the present study, we investigated the effect of uPA gene modified-BMSCs on CCl4-induced liver fibrosis in rats and the mechanism by which uPA-BMSCs ameliorate fibrosis. These data show that administration of uPA gene modified-BMSCs resulted in a further improvement of liver fibrosis than BMSCs alone. Extensive evidence supports the fact that the plasminogen activation system participates in the matrix remodeling process, and alternative expression of the plasminogen activation system was found in fibrotic organs. Until now, the anti-fibrotic activity of uPA was confirmed in animal models of liver fibrosis[41,42]. So we believe that a combination of BMSCs transplantation and uPA gene therapy may provide a novel tool for the treatment of liver fibrosis. In the present study, we chose the classical method by injecting with CCl4 subcutaneously for 8 wk to develop the liver fibrosis rat model. Administration of CCl4 to rodents is widely used to study the therapeutic method for hepatic injury. The reason for selecting CCl4 was that CCl4-induced fibrosis in rats shares similar pathological changes to human liver fibrosis that is characterized by centrilobular necrosis followed by hepatic fibrosis. According to our results, the uPA-BMSCs group had lower serum levels of ALT, AST, and TBIL, and higher ALB levels than the model or BMSCs group. Moreover, serum levels of HA, LN, and PCIII, which reflect ECM deposition, were significantly lower in the uPA-BMSCs group than in the model group or BMSCs group. Histological examination also indicated that uPA-BMSCs treatment remarkably reduced the deposition of collagen fibers compared with the model group or BMSCs group. These findings were also observed by Sun et al[43] who suggested that transplantation of uPA gene modified cell could suppress hepatic fibrosis and ameliorate liver function. As mentioned above, a therapeutic effect of uPA gene modified BMSCs in liver fibrosis has been established more thoroughly than single BMSCs transplantation, and it may be a more favorable therapeutic option than BMSCs alone. As a result, the present study deduced the reason that increased expression of uPA gene not only improved degradation of ECM components directly or indirectly by activating the MMPs, but also possibly enhanced the transplantation of BMSCs by participating in cell proliferation, adhesion, migration, and angiogenesis in a plasmin-independent manner[44]. However, the precise molecular mechanism remained to be defined and further investigated.
HSCs have been regarded as the main cells for synthesizing and secreting ECM components. Inhibition of the activation of HSCs has become an important treatment strategy for liver fibrosis. It has been proven that the Wnt signaling pathway is related to HSCs activation and fibrogenesis[45,46]. It was found that inhibition of Wnt/β-catenin signaling resulted in the down-regulation of HSC activation and attenuated CCl4-induced liver fibrosis eventually[24,25]. Hence, we assumed that the anti-fibrotic activity of uPA may be related to the down-regulation of the Wnt signaling pathway. In the present study, we investigated the effect of uPA-BMSCs on the expression of molecules of the Wnt signaling pathway by qRT-PCR and western blot. Results showed that the Wnt signaling pathway was abnormally activated in the model group, and uPA-BMSCs treatment can down-regulate the expression of molecules of the Wnt signaling pathway. Both significantly lower mRNA levels and protein levels of β-catenin, Wnt4 and Wnt5a in liver tissues were observed in the uPA-BMSCs treatment group compared with the model group. To some degree, we also concluded that uPA-BMSCs attenuated the development of liver fibrosis possibly partly by down-regulating the Wnt signaling pathway. To the best of our knowledge, the present study is the first attempt to report the potential association between uPA and the Wnt signaling pathway.
In conclusion, the present study displayed that uPA gene modified BMSCs significantly improved liver function and attenuated CCl4-induced liver fibrosis, thus providing a new and efficient approach for the treatment of liver fibrosis by enhancing uPA expression to improve ECM degradation. Furthermore, it also resulted in decreased mRNA and protein expression of molecules involved in Wnt signaling, suggesting that it is antifibrotic partly due to the down-regulation of the Wnt signaling pathway. As the molecular mechanism involved in liver fibrosis was complicated, it needed to be further explored. While all of these conclusions are consistent with our data, the mechanisms by which uPA-BMSCs transplantation inhibits liver fibrosis remain to be defined. Thus, further studies should be carried out to support our findings here. What’s more, future research on the safety and efficacy of uPA-BMSCs therapy in liver fibrosis is also needed to optimize this approach for clinical applications that could be used to treat liver cirrhosis patients. Our current study may provide a foundation for designing therapeutic regimens for inhibiting the progression of chronic liver diseases in clinical settings.
The authors would like to thank the members of Experimental Animal Center and Clinical Trial Service Unit of Guangxi Medical University for their technical support. All authors also wish to express their gratitude to the medical personnel of Department of Gastroenterology and Clinical Experimental Medicine, the First Affiliated Hospital of Guangxi Medical University for their theoretical guidance.
Bone marrow-derived mesenchymal stem cells (BMSCs) have been reported to be associated with the treatment of liver fibrosis. However, some reports indicated that the therapeutic effect of simple BMSCs therapy has been limited. Therefore, the genetic modification of BMSCs might be a more promising option for improving their therapeutic potential. Little is known about the therapeutic effects of human urokinase-type plasminogen activator (uPA) gene-modified BMSCs on liver fibrosis.
Recently, it has been reported that uPA is a specific serine protease which plays an important role in extracellular matrix (ECM) degradation. Little is known about the therapeutic effects of human uPA gene-modified BMSCs on liver fibrosis. Therefore, uPA gene-modified BMSCs may be a novel therapy to treat liver fibrosis.
This study displayed that uPA gene modified BMSCs significantly improved liver function and attenuated CCl4-induced liver fibrosis. Furthermore, it also resulted in decreased mRNA and protein expression of molecules involved in Wnt signaling, suggesting that it is antifibrotic partly due to the down-regulation of the Wnt signaling pathway.
This study provides a foundation for designing therapeutic regimens for inhibiting the progression of chronic liver diseases in clinical settings. Meanwhile, uPA-BMSCs therapy in liver fibrosis is hopeful to be optimized for clinical applications that could be used to treat liver cirrhosis patients.
BMSCs, non-hematopoietic cells with multi-lineage potential, can differentiate into multiple mature cell phenotypes in vitro, including adipocytes, osteocytes, chondrocytes and so on. They are used for studies of stem cell differentiation, tissue engineering, cell and gene therapy, and have potential future clinical applications.
In this study, BMSCs transfected with Ad-uPA were transplanted into rats with CCl4-induced liver fibrosis, to evaluate a possible therapeutic approach for treatment of liver fibrosis. The results revealed that uPA gene apparently was capable of BMSC modification by suppressing liver fibrosis through down-regulation of the Wnt signaling pathway. This well designed and executed study with an animal model of liver fibrosis, provides clear benefits of possible “gene therapy” in treatment of liver fibrosis. In general, the report is well written and the results are supported by the experimental data.
Biostatistics statement: The statistical methods of this study were reviewed by Ji-Qiao Xiang from the Guangxi Medical University.
P- Reviewer: Slomiany BL S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Wang B, Li W, Chen Y, Wang Y, Sun C, Chen Y, Lu H, Fan J, Li D. Coexpression of Smad7 and UPA attenuates carbon tetrachloride-induced rat liver fibrosis. Med Sci Monit. 2012;18:BR394-BR401. [PubMed] [Cited in This Article: ] |
| 2. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2084] [Cited by in F6Publishing: 2047] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 3. | Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1417] [Cited by in F6Publishing: 1319] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 4. | Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1203] [Cited by in F6Publishing: 1150] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 5. | Dong S, Su SB. Advances in mesenchymal stem cells combined with traditional Chinese medicine therapy for liver fibrosis. J Integr Med. 2014;12:147-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Yao X, Zhou N, Wan L, Su X, Sun Z, Mizuguchi H, Yoshioka Y, Nakagawa S, Zhao RC, Gao JQ. Polyethyleneimine-coating enhances adenoviral transduction of mesenchymal stem cells. Biochem Biophys Res Commun. 2014;447:383-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Shao CH, Chen SL, Dong TF, Chai H, Yu Y, Deng L, Wang Y, Cheng F. Transplantation of bone marrow-derived mesenchymal stem cells after regional hepatic irradiation ameliorates thioacetamide-induced liver fibrosis in rats. J Surg Res. 2014;186:408-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kim MD, Kim SS, Cha HY, Jang SH, Chang DY, Kim W, Suh-Kim H, Lee JH. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med. 2014;46:e110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Kumar S, Chanda D, Ponnazhagan S. Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther. 2008;15:711-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Salgado S, Garcia J, Vera J, Siller F, Bueno M, Miranda A, Segura A, Grijalva G, Segura J, Orozco H. Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy. Mol Ther. 2000;2:545-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Leyland H, Gentry J, Arthur MJ, Benyon RC. The plasminogen-activating system in hepatic stellate cells. Hepatology. 1996;24:1172-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Bezerra JA, Currier AR, Melin-Aldana H, Sabla G, Bugge TH, Kombrinck KW, Degen JL. Plasminogen activators direct reorganization of the liver lobule after acute injury. Am J Pathol. 2001;158:921-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 758] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 14. | Shanmukhappa K, Sabla GE, Degen JL, Bezerra JA. Urokinase-type plasminogen activator supports liver repair independent of its cellular receptor. BMC Gastroenterol. 2006;6:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Pohl JF, Melin-Aldana H, Sabla G, Degen JL, Bezerra JA. Plasminogen deficiency leads to impaired lobular reorganization and matrix accumulation after chronic liver injury. Am J Pathol. 2001;159:2179-2186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, Tsukamoto H. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G39-G49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 17. | Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3831] [Cited by in F6Publishing: 3935] [Article Influence: 207.1] [Reference Citation Analysis (0)] |
| 18. | Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25:254-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 362] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 19. | Kikuchi A, Yamamoto H, Sato A, Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol. 2011;291:21-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Clark CE, Nourse CC, Cooper HM. The tangled web of non-canonical Wnt signalling in neural migration. Neurosignals. 2012;20:202-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 22. | Andersen P, Uosaki H, Shenje LT, Kwon C. Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol. 2012;22:257-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Ge WS, Wang YJ, Wu JX, Fan JG, Chen YW, Zhu L. β-catenin is overexpressed in hepatic fibrosis and blockage of Wnt/β-catenin signaling inhibits hepatic stellate cell activation. Mol Med Rep. 2014;9:2145-2151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Li W, Zhu C, Chen X, Li Y, Gao R, Wu Q. Pokeweed antiviral protein down-regulates Wnt/β-catenin signalling to attenuate liver fibrogenesis in vitro and in vivo. Dig Liver Dis. 2011;43:559-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Kordes C, Sawitza I, Häussinger D. Canonical Wnt signaling maintains the quiescent stage of hepatic stellate cells. Biochem Biophys Res Commun. 2008;367:116-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Xiong WJ, Hu LJ, Jian YC, Wang LJ, Jiang M, Li W, He Y. Wnt5a participates in hepatic stellate cell activation observed by gene expression profile and functional assays. World J Gastroenterol. 2012;18:1745-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Ikegami T, Honda A, Miyazaki T, Bouscarel B, Rojkind M, Hyodo I, Matsuzaki Y. Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology. 2006;44:612-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Chevallier M, Guerret S, Chossegros P, Gerard F, Grimaud JA. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology. 1994;20:349-355. [PubMed] [Cited in This Article: ] |
| 29. | Baron C, Somogyi R, Greller LD, Rineau V, Wilkinson P, Cho CR, Cameron MJ, Kelvin DJ, Chagnon P, Roy DC. Prediction of graft-versus-host disease in humans by donor gene-expression profiling. PLoS Med. 2007;4:e23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Alvaro Mercadal B, Imbert R, Demeestere I, Gervy C, De Leener A, Englert Y, Costagliola S, Delbaere A. AMH mutations with reduced in vitro bioactivity are related to premature ovarian insufficiency. Hum Reprod. 2015;30:1196-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1329] [Cited by in F6Publishing: 1362] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 32. | Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 681] [Cited by in F6Publishing: 685] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 33. | Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [PubMed] [Cited in This Article: ] |
| 35. | Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 36. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 37. | Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83-88. [PubMed] [Cited in This Article: ] |
| 38. | Carvalho AB, Quintanilha LF, Dias JV, Paredes BD, Mannheimer EG, Carvalho FG, Asensi KD, Gutfilen B, Fonseca LM, Resende CM. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells. 2008;26:1307-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955-963. [PubMed] [Cited in This Article: ] |
| 41. | Piotrowski WJ, Górski P, Pietras T, Fendler W, Szemraj J. The selected genetic polymorphisms of metalloproteinases MMP2, 7, 9 and MMP inhibitor TIMP2 in sarcoidosis. Med Sci Monit. 2011;17:CR598-CR607. [PubMed] [Cited in This Article: ] |
| 42. | Bueno M, Salgado S, Beas-Zárate C, Armendariz-Borunda J. Urokinase-type plasminogen activator gene therapy in liver cirrhosis is mediated by collagens gene expression down-regulation and up-regulation of MMPs, HGF and VEGF. J Gene Med. 2006;8:1291-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Sun C, Li DG, Chen YW, Chen YW, Wang BC, Sun QL, Lu HM. Transplantation of urokinase-type plasminogen activator gene-modified bone marrow-derived liver stem cells reduces liver fibrosis in rats. J Gene Med. 2008;10:855-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004;25:450-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006;45:401-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223-229. [PubMed] [Cited in This Article: ] |