Copyright
©The Author(s) 2016.
World J Gastroenterol. Dec 28, 2016; 22(48): 10631-10642
Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10631
Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10631
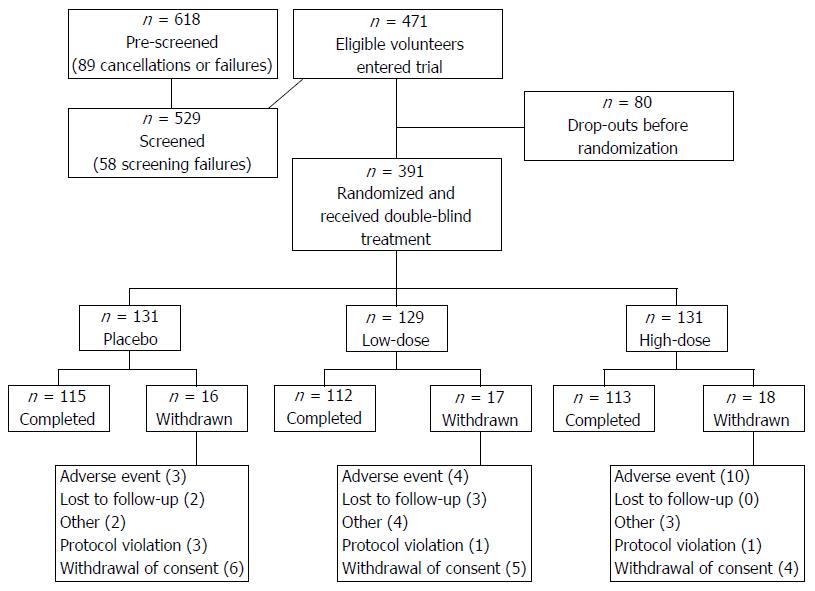
Figure 2 Disposition of volunteers.
During the 8-wk run-in period prior to randomization, 17% of eligible volunteers dropped out. After randomization, most volunteers (87%) completed the trial. Withdrawals were evenly distributed between treatment groups. Low- and high-dose treatment groups received 109 or 1010 CFU Lactobacillus acidophilus NCFM daily.
- Citation: Lyra A, Hillilä M, Huttunen T, Männikkö S, Taalikka M, Tennilä J, Tarpila A, Lahtinen S, Ouwehand AC, Veijola L. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol 2016; 22(48): 10631-10642
- URL: https://www.wjgnet.com/1007-9327/full/v22/i48/10631.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i48.10631