Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8798
Peer-review started: June 16, 2016
First decision: July 29, 2016
Revised: August 15, 2016
Accepted: August 30, 2016
Article in press: August 30, 2016
Published online: October 21, 2016
To determine whether cyclooxygenase-2 (COX-2) and prostaglandin E1 receptor (EP1) contribute to disease and whether they help predict prognosis.
We retrospectively reviewed the records of 116 patients with hepatocellular carcinoma (HCC) who underwent surgery between 2008 and 2011 at our hospital. Expression of COX-2 and EP1 receptor was examined by immunohistochemistry of formalin-fixed, paraffin-embedded tissues using polyclonal antibodies. Possible associations between immunohistochemical scores and survival were determined.
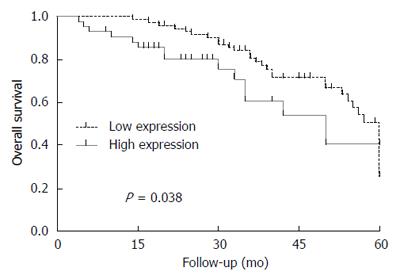
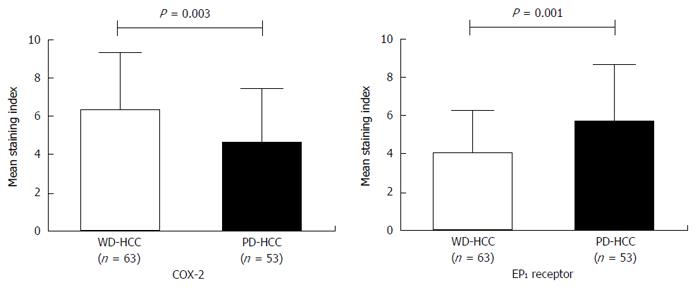
Factors associated with poor overall survival (OS) were alpha-fetoprotein > 400 ng/mL, tumor size ≥ 5 cm, and high EP1 receptor expression, but not high COX-2 expression. Disease-free survival was not significantly different between patients with low or high levels of COX-2 or EP1. COX-2 immunoreactivity was significantly higher in well-differentiated HCC tissues (Edmondson grade I-II) than in poorly differentiated tissues (Edmondson grade III-IV) (P = 0.003). EP1 receptor immunoreactivity was significantly higher in poorly differentiated tissue than in well-differentiated tissue (P = 0.001).
COX-2 expression appears to be linked to early HCC events (initiation), while EP1 receptor expression may participate in tumor progression and predict survival.
Core tip: We retrospectively reviewed the records of 116 patients with hepatocellular carcinoma (HCC) who underwent surgery between 2008 and 2011 at our hospital. Our results suggest that the factors associated with poor overall survival were alpha-fetoprotein > 400 ng/mL, tumor size ≥ 5 cm, and high prostaglandin E1 (EP1) receptor expression, but not high cyclooxygenase-2 (COX-2) expression. Disease-free survival did not differ significantly between patients with low or high levels of COX-2 or EP1. COX-2 immunoreactivity was significantly higher in well-differentiated HCC tissues (Edmondson grade I-II) than in poorly differentiated tissues (Edmondson grade III-IV) (P = 0.003). EP1 receptor immunoreactivity was significantly higher in poorly differentiated tissue than in well-differentiated tissue (P = 0.001).
- Citation: Yang HJ, Jiang JH, Yang YT, Yang XD, Guo Z, Qi YP, Zeng FH, Zhang KL, Chen NZ, Xiang BD, Li LQ. Cyclooxygenase-2 expression is associated with initiation of hepatocellular carcinoma, while prostaglandin receptor-1 expression predicts survival. World J Gastroenterol 2016; 22(39): 8798-8805
- URL: https://www.wjgnet.com/1007-9327/full/v22/i39/8798.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i39.8798
Hepatocellular carcinoma (HCC) is one of the most aggressive tumors and the third most frequent cause of cancer-related death in the world[1,2]. Although early diagnosis and treatment of HCC have improved substantially, prognosis remains unsatisfactory. HCC often involves highly malignant tumors that respond poorly or not at all to adjuvant systemic and local therapies. This highlights the need for new approaches to prevent and treat the disease.
Two molecules that may be involved in HCC at different stages, and that therefore may be useful for understanding the pathogenesis and progression of the disease, are cyclooxygenase-2 (COX-2) and prostaglandin E1 receptor (EP1 receptor). These molecules have already been shown to play important roles in the onset of various cancers, including HCC[3-9].
COX-2 inhibits apoptosis and increases proliferation in various type of tumors[10-12]. It also triggers the production of vascular endothelial growth factor and activates metalloproteinases, substantially altering the tumor microenvironment of various cancers[13-15]. The precise role of COX-2 in HCC remains unclear. Its expression decreases with extent of de-differentiation, and it does not appear to be associated with prognosis[16-19]. It may be involved in HCC initiation, although direct evidence of this is lacking.
COX-2 catalyzes the conversion of arachidonic acid to prostaglandin E2, which promotes the progression of various types of tumors by binding to the G-protein-coupled EP1 receptor. This led us to wonder whether EP1 receptor expression might correlate with HCC progression and might even serve as a prognostic indicator of survival. In fact, in a mouse model of chemically induced colon cancer, administration of selective EP1 receptor antagonists or knockout of the EP1 receptor gene led to nearly 60% fewer precancerous lesions and a lower overall colon cancer incidence[20,21]. EP1 receptor antagonists have also been reported to block the progression of other types of tumor[22,23], including HCC[18,24].
To begin to clarify the potential roles of COX-2 and EP1 receptor in HCC, and to determine the potential prognostic value of EP1 receptor expression, we examined relative expression levels in tissues taken from HCC patients treated at our hospital, and correlated these levels with survival.
This research was approved by the Ethics Committee of the Tumor Hospital of Guangxi Medical University, and patients provided informed consent for their data and tissue to be used for research purposes when they were admitted for treatment at our hospital.
This study was a retrospective analysis of HCC patients treated by curative hepatectomy at the Tumor Hospital of Guangxi Medical University between May 2008 and May 2011. To be included in our study, patients needed to have pathology-confirmed HCC and no history of antitumor therapies before hepatic resection. They also needed to satisfy the following curative hepatectomy criteria: (1) the tumor removed by hepatectomy was solitary; (2) the surgery margin was greater than 1 cm; (3) there was no residual tumor[25], portal tumor thromboses[26] or extrahepatic metastases based on post-surgical imaging; and (4) patients with high levels of alpha-fetoprotein (AFP) before surgery had normal levels within two months after surgery. Patients were excluded from the study if they had multiple tumors, extrahepatic metastases or macroscopic intrahepatic metastases adjacent to the primary tumor.
All HCC patients were followed up 1 mo after resection, then at 3-mo intervals in the first year, and then at 3-6 mo intervals thereafter until 60 mo after resection or death. During each follow-up visit, routine investigations including AFP level, liver function, chest X-ray, ultrasound, CT or MRI were conducted.
Tumor specimens were fixed in 10% formalin, embedded in paraffin, cut into 3-μm sections, de-paraffinized with xylene and rehydrated by decreasing concentrations of ethanol. Antigen retrieval was performed for 10 min at 95 °C in citrate buffer (pH 6.0) in a microwave oven. Sections were immersed in 3% hydrogen peroxide for 15 min to block endogenous peroxidases, then incubated at 37 °C for 1 h with rabbit anti-human COX-2 polyclonal antibody (1:400; Abcam, United Kingdom) or rabbit anti-human EP1 receptor polyclonal antibody (1:200; Abcam). The sections were rinsed in phosphate-buffered saline (PBS), incubated with biotinylated anti-rabbit immunoglobulin for 20 min at room temperature, and then rinsed again with PBS. The sections were incubated with anti-horseradish peroxidase conjugate for 10 min, rinsed with PBS, and incubated with diaminobenzidine for 10 min. Finally, the sections were counterstained with hematoxylin. As a negative control, tissues were treated as described above, except they were incubated with PBS instead of primary antibodies.
Immunohistochemical staining results were independently evaluated by three authors (Hao-Jie Yang, Zhe Guo and Yu-Ting Yang) and an experienced hepatopathologist (Chun-Jun Li) from the Department of Pathology of Guangxi Tumor Hospital. The percentages of cells positive for COX-2 or EP1 receptor as well as relative staining intensity were determined. Percentages of positive cells were categorized as follows: 0 (no positive tumor cells), 1 (1%-25% positive), 2 (26%-50% positive), 3 (51%-75% positive), and 4 (76%-100% positive). Staining intensity was categorized as follows: 0 (no staining), 1 (weak, light yellow), 2 (moderate, yellow-brown), and 3 (strong, brown)[27]. The scores for positive cell percentages and for staining intensity were multiplied together to yield a single immunohistochemical staining index from 0 to 12. Sections with an index of 0-5 were defined as showing low expression, while those with an index of 6-12 were defined as showing high expression.
All statistical analyses were performed using SPSS 19.0 (IBM, United States). Inter-group differences in categorical variables were assessed for significance using the χ2 test; differences in continuous variables were assessed using the Mann-Whitney U test or t-test. OS and DFS were analyzed using the Kaplan-Meier method, and differences between curves were assessed for significance using the log-rank test. Multivariate Cox proportional hazards modeling was used to identify independent prognostic factors. The threshold for significance was defined as P < 0.05.
During the study, 748 patients with HCC were scheduled for hepatectomy in my center. Of these, 221 (29.5%) were excluded as they had received initial HCC treatment in other hospitals. Of the remaining 527 patients, 161 (30.5%) had solitary nodular tumors without portal tumor thromboses or extrahepatic metastases. We excluded 33 (20.4%) because they underwent only transarterial chemoembolization, local ablation therapy, or ethanol injection, and we excluded 12 (7.4%) as they lacked complete follow-up data. In total, 116 (72%) patients were included in the final analysis (93 men, 23 women) with a median age of 67 (range, 39-83) (Table 1). Immunohistochemistry showed low COX-2 expression in 62 patients (53.4%) and low EP1 receptor expression in 73 (62.9%).
| Variable | COX-2 | P value | EP1 receptor | P value | ||
| Low expression | High expression | Low expression | High expression | |||
| (n = 62) | (n = 54) | (n = 73) | (n = 43) | |||
| Gender, M/F | 48/14 | 45/9 | 0.426 | 60/13 | 33/10 | 0.477 |
| Age (yr) | 46.9 ± 10.8 | 47.9 ± 11.5 | 0.308 | 46.6 ± 11.1 | 47.3 ± 11.2 | 0.486 |
| HbsAg | ||||||
| Negative | 10 | 10 | 0.734 | 11 | 9 | 0.420 |
| Positive | 52 | 44 | 62 | 34 | ||
| Liver cirrhosis | ||||||
| No | 10 | 10 | 0.734 | 14 | 6 | 0.472 |
| Yes | 52 | 44 | 59 | 37 | ||
| AFP (ng/mL) | ||||||
| < 400 | 41 | 44 | 0.062 | 56 | 29 | 0.276 |
| ≥ 400 | 21 | 10 | 17 | 14 | ||
| Edmondson grade | ||||||
| I-II | 26 | 37 | 0.004 | 46 | 17 | 0.014 |
| III–IV | 36 | 17 | 27 | 26 | ||
| Child-Pugh class | ||||||
| A | 43 | 48 | 0.773 | 60 | 31 | 0.201 |
| B | 11 | 14 | 13 | 12 | ||
| Tumor capsule | ||||||
| Complete | 37 | 34 | 0.717 | 44 | 27 | 0.788 |
| Incomplete | 25 | 20 | 29 | 16 | ||
| Tumor size (cm) | 5.7 (3-7) | 6.6 (4-8.6) | 0.134 | 5 (3.5-7) | 6 (3.7-8.5) | 0.207 |
| Albumin (g/L) | 41.1 ± 4.4 | 40.4 ± 4.6 | 0.434 | 41.5 ± 4.3 | 40.2 ± 4.5 | 0.134 |
| Platelet count (109/L) | 167.9 (107.3-205.3 | 186.6 (136.3-230) | 0.136 | 178.9 (107.3-205.3 | 186.6 (136.3-230) | 0.833 |
| AST (U/L) | 56.6 (28.3-61) | 55.6 (27-60) | 0.924 | 38 (27-60) | 44 (30.5-61.5) | 0.737 |
| ALT (U/L) | 54.8 (28-56) | 62.7 (24.8-57.9) | 0.539 | 37(27-56) | 38(27-55) | 0.917 |
| Total bilirubin (μmol/L) | 14.08 (8.9-18.4) | 14.3 (9.4-15.3) | 0.912 | 11.9(9.35-16.6) | 13.4(9-17.7) | 0.580 |
OS was significantly lower among patients with high expression of EP1 receptor than among those with low expression (Figure 1). OS did not differ significantly between patients with low or high COX-2 expression. DFS did not differ significantly between patients with low or high expression of COX-2 or EP1 receptor.
The Cox hazards model showed 3 independent predictors of poor OS: tumor size ≥ 5 cm, high expression of EP1 receptor and AFP ≥ 400 ng/mL (Table 2).
| Factors | Hazard ratio | 95%CI | P value |
| AFP ≥ 400 ng/mL | 1.691 | 1.094-2.614 | 0.018 |
| Incomplete tumor capsule | 0.979 | 0.659-1.454 | 0.915 |
| Tumor size ≥ 5 cm | 1.582 | 1.027-2.438 | 0.038 |
| Edmondson grade III–IV | 1.149 | 0.663-1.992 | 0.621 |
| EP1 receptor expression | 2.318 | 1.190-4.516 | 0.014 |
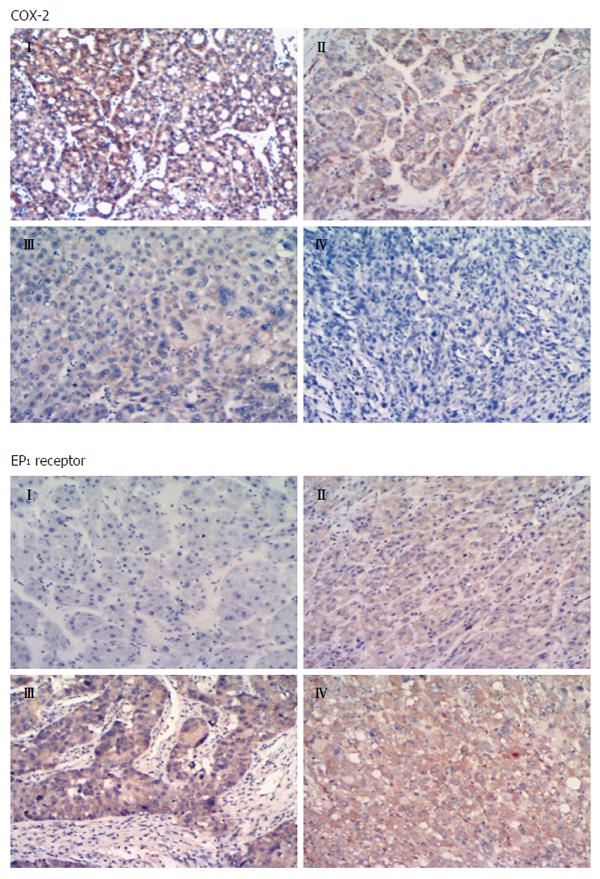
COX-2 immunoreactivity was significantly higher in well-differentiated HCC tissues (Edmondson grade I-II) than in poorly differentiated tissues (Edmondson grade III-IV) (P = 0.003). EP1 receptor immunoreactivity was significantly higher in poorly differentiated tissue than in well-differentiated tissue (Figure 2; P = 0.001). Figure 3 shows representative examples of different staining results in tissues of different histology grade.
HCC is one of the most aggressive tumors and has a poor prognosis. Some patients can undergo curative resection, but this treatment is associated with a high rate of intrahepatic recurrence. Most patients with HCC are ineligible for resection because their disease has already reached an advanced stage by the time it is diagnosed. These patients are treated with local or systemic adjuvant modalities that provide only short-term regression, stabilization, or symptomatic control. Therefore, new therapeutic strategies are needed to improve long-term survival.
Our results indicate that the EP1 receptor is involved in HCC progression, suggesting that it may be a reasonable therapeutic target. High expression of the EP1 receptor was associated with poor prognosis in our patients, and expression was significantly higher in poorly differentiated tissue than in well-differentiated tissue. The observed correlation between higher expression and poorer differentiation is consistent with a previous study[18]. These results suggest that targeting the EP1 receptor may provide a more selective approach to treating HCC than using COX inhibitors to block prostaglandin E2 synthesis, which increases the risk of cardiovascular events[28].
Although studies have associated COX-2 expression with differentiation, invasion and metastasis in HCC[3,18,24], it has not been linked with survival. In the present study, we failed to find an association between COX-2 expression and survival when patients were dichotomized into groups with low or high expression. While it is possible that a more quantitative approach may identify associations between COX-2 levels and survival, we believe it is more likely that expression of COX-2 may be important only during initiation of HCC, whereas the EP1 receptor, which is the downstream target of prostaglandin E2 generated by COX-2, may be involved in disease progression. This may explain why we observed a different relationship between the expression of COX-2 and EP1 receptor: patients with high expression of one showed low expression of the other. This may also explain why we found that expression of the EP1 receptor, but not the COX-2 receptor, predicted OS in our cohort.
Several factors may help to explain why high expression of the EP1 receptor predicts poor survival. The receptor enhances tumor cell proliferation, invasion and migration[4,6,29,30], as well as adaptation to hypoxic conditions[6,31]. The receptor has also been reported to inhibit immune function and promote tumor progression[32]. The EP1 receptor can even induce prostaglandin E2 production by binding to the receptor of Fas ligand[32-34].
This study has some limitations. Firstly, the authors used only an HE method, therefore more accurate and quantitative methods, such as Western blot, polymerase chain reaction etc., should be applied in future studies. Secondly, it is known that other cytokines, except COX-2 and EP1, have been reported to be strongly associated with HCC. These cytokines may be involved in different aspects of the pathogenesis of HCC and should be explored as a network pattern in future studies.
In conclusion, our results suggest that COX-2 expression correlates with an early event during the initiation of HCC, while EP1 receptor expression plays an important role in tumor progression and predicts OS.
Hepatocellular carcinoma (HCC) is one of the most aggressive tumors and the third most frequent cause of cancer-related death in the world. Although early diagnosis and treatment of HCC have improved substantially, prognosis remains unsatisfactory. HCC often involves highly malignant tumors that respond poorly or not at all to adjuvant systemic and local therapies. This highlights the need for new approaches to prevent and treat the disease.
Two molecules that may be involved in HCC at different stages, and that therefore may be useful for understanding the pathogenesis and progression of the disease, are cyclooxygenase-2 (COX-2) and prostaglandin E1 receptor (EP1 receptor). These molecules have already been shown to play important roles in the onset of various cancers, including HCC.
The authors retrospectively reviewed the records of 116 patients with HCC who underwent surgery between 2008 and 2011 at their hospital, and found that COX-2 expression appears to be linked to early HCC events (initiation), while EP1 receptor expression may participate in tumor progression and predict survival.
These results suggest that targeting the EP1 receptor may provide a more selective approach to treating HCC than using COX inhibitors to block prostaglandin E2 synthesis, which increases the risk of cardiovascular events.
Percentages of positive cells were categorized as follows: 0 (no positive tumor cells), 1 (1%-25% positive), 2 (26%-50% positive), 3 (51%-75% positive), and 4 (76%-100% positive). Staining intensity was categorized as follows: 0 (no staining), 1 (weak, light yellow), 2 (moderate, yellow-brown), and 3 (strong, brown). The scores for positive cell percentages and for staining intensity were multiplied together to yield a single immunohistochemical staining index from 0 to 12. Sections with an index of 0-5 were defined as showing low expression, while those with an index of 6-12 were defined as showing high expression.
The paper is a good study on COX-2 and EP1 receptor immunoreactivity in patients with HCC. The investigators shown that COX-2 immunoreactivity was higher in well-differentiated HCC tissues and EP1 receptor immunoreactivity was significantly higher in poorly differentiated tissue than in well-differentiated tissue.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Alexopoulou A, de Oliveira CPMS, Mihaila RG S- Editor: Gong ZM L- Editor: Webster JR E- Editor: Wang CH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 20893] [Article Influence: 2321.4] [Reference Citation Analysis (2)] |
| 2. | Yang HJ, Guo Z, Yang YT, Jiang JH, Qi YP, Li JJ, Li LQ, Xiang BD. Blood neutrophil-lymphocyte ratio predicts survival after hepatectomy for hepatocellular carcinoma: A propensity score-based analysis. World J Gastroenterol. 2016;22:5088-5095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Guo Z, Jiang JH, Zhang J, Yang HJ, Yang FQ, Qi YP, Zhong YP, Su J, Yang RR, Li LQ. COX-2 Promotes Migration and Invasion by the Side Population of Cancer Stem Cell-Like Hepatocellular Carcinoma Cells. Medicine (Baltimore). 2015;94:e1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Zhang H, Cheng S, Zhang M, Ma X, Zhang L, Wang Y, Rong R, Ma J, Xia S, Du M. Prostaglandin E2 promotes hepatocellular carcinoma cell invasion through upregulation of YB-1 protein expression. Int J Oncol. 2014;44:769-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Jin J, Chang Y, Wei W, He YF, Hu SS, Wang D, Wu YJ. Prostanoid EP1 receptor as the target of (-)-epigallocatechin-3-gallate in suppressing hepatocellular carcinoma cells in vitro. Acta Pharmacol Sin. 2012;33:701-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Kim SH, Park YY, Kim SW, Lee JS, Wang D, DuBois RN. ANGPTL4 induction by prostaglandin E2 under hypoxic conditions promotes colorectal cancer progression. Cancer Res. 2011;71:7010-7020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Bai XM, Jiang H, Ding JX, Peng T, Ma J, Wang YH, Zhang L, Zhang H, Leng J. Prostaglandin E2 upregulates survivin expression via the EP1 receptor in hepatocellular carcinoma cells. Life Sci. 2010;86:214-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol. 2006;207:261-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol Cancer Ther. 2004;3:1031-1039. [PubMed] [Cited in This Article: ] |
| 10. | Zhang P, Luo HS, Li M, Tan SY. Artesunate inhibits the growth and induces apoptosis of human gastric cancer cells by downregulating COX-2. Onco Targets Ther. 2015;8:845-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Qian M, Yang X, Li Z, Jiang C, Song D, Yan W, Liu T, Wu Z, Kong J, Wei H. P50-associated COX-2 extragenic RNA (PACER) overexpression promotes proliferation and metastasis of osteosarcoma cells by activating COX-2 gene. Tumour Biol. 2016;37:3879-3886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Lv P, Zhang P, Li X, Chen Y. Micro ribonucleic acid (RNA)-101 inhibits cell proliferation and invasion of lung cancer by regulating cyclooxygenase-2. Thorac Cancer. 2015;6:778-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Piao MS, Choi JY, Yun SJ, Lee JB, Lee SC. Up-regulation of cyclooxygenase 2 and matrix metalloproteinases-2 and -9 in cutaneous squamous cell carcinoma: active role of inflammation and tissue remodeling in carcinogenesis. Ann Dermatol. 2013;25:145-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Mohammad MA, Zeeneldin AA, Abd Elmageed ZY, Khalil EH, Mahdy SM, Sharada HM, Sharawy SK, Abdel-Wahab AH. Clinical relevance of cyclooxygenase-2 and matrix metalloproteinases (MMP-2 and MT1-MMP) in human breast cancer tissue. Mol Cell Biochem. 2012;366:269-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Li Y, Li S, Sun D, Song L, Liu X. Expression of 15-hydroxyprostaglandin dehydrogenase and cyclooxygenase-2 in non-small cell lung cancer: Correlations with angiogenesis and prognosis. Oncol Lett. 2014;8:1589-1594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Yang Y, Zhu J, Gou H, Cao D, Jiang M, Hou M. Clinical significance of Cox-2, Survivin and Bcl-2 expression in hepatocellular carcinoma (HCC). Med Oncol. 2011;28:796-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Yildirim Y, Ozyilkan O, Bilezikci B, Akcali Z, Haberal M. Lack of influence of cyclooxygenese-2 expression in hepatocellular carcinomas on patient survival. Asian Pac J Cancer Prev. 2008;9:295-298. [PubMed] [Cited in This Article: ] |
| 18. | Breinig M, Rieker R, Eiteneuer E, Wertenbruch T, Haugg AM, Helmke BM, Schirmacher P, Kern MA. Differential expression of E-prostanoid receptors in human hepatocellular carcinoma. Int J Cancer. 2008;122:547-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 296] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Narumiya S, Sugimura T. Inhibitory effect of a prostaglandin E receptor subtype EP(1) selective antagonist, ONO-8713, on development of azoxymethane-induced aberrant crypt foci in mice. Cancer Lett. 2000;156:57-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Ushikubi F, Narumiya S. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59:5093-5096. [PubMed] [Cited in This Article: ] |
| 22. | Kawamori T, Uchiya N, Nakatsugi S, Watanabe K, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Sugimura T, Wakabayashi K. Chemopreventive effects of ONO-8711, a selective prostaglandin E receptor EP(1) antagonist, on breast cancer development. Carcinogenesis. 2001;22:2001-2004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Tober KL, Wilgus TA, Kusewitt DF, Thomas-Ahner JM, Maruyama T, Oberyszyn TM. Importance of the EP(1) receptor in cutaneous UVB-induced inflammation and tumor development. J Invest Dermatol. 2006;126:205-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Cusimano A, Foderà D, Lampiasi N, Azzolina A, Notarbartolo M, Giannitrapani L, D’Alessandro N, Montalto G, Cervello M. Prostaglandin E2 receptors and COX enzymes in human hepatocellular carcinoma: role in the regulation of cell growth. Ann N Y Acad Sci. 2009;1155:300-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 553] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 26. | Xia Y, Qiu Y, Li J, Shi L, Wang K, Xi T, Shen F, Yan Z, Wu M. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol. 2010;17:3137-3144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Baker AM, Bird D, Welti JC, Gourlaouen M, Lang G, Murray GI, Reynolds AR, Cox TR, Erler JT. Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis. Cancer Res. 2013;73:583-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Bai X, Wang J, Zhang L, Ma J, Zhang H, Xia S, Zhang M, Ma X, Guo Y, Rong R. Prostaglandin E2 receptor EP1-mediated phosphorylation of focal adhesion kinase enhances cell adhesion and migration in hepatocellular carcinoma cells. Int J Oncol. 2013;42:1833-1841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Yang SF, Chen MK, Hsieh YS, Chung TT, Hsieh YH, Lin CW, Su JL, Tsai MH, Tang CH. Prostaglandin E2/EP1 signaling pathway enhances intercellular adhesion molecule 1 (ICAM-1) expression and cell motility in oral cancer cells. J Biol Chem. 2010;285:29808-29816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683-6691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | O’Callaghan G, Ryan A, Neary P, O’Mahony C, Shanahan F, Houston A. Targeting the EP1 receptor reduces Fas ligand expression and increases the antitumor immune response in an in vivo model of colon cancer. Int J Cancer. 2013;133:825-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Liu Q, Zhang M, Yu Y, Liu X, Cao X. Fas signal promotes lung cancer growth by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2. J Immunol. 2009;182:3801-3808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | O’Callaghan G, Kelly J, Shanahan F, Houston A. Prostaglandin E2 stimulates Fas ligand expression via the EP1 receptor in colon cancer cells. Br J Cancer. 2008;99:502-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |