Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7613
Revised: June 9, 2016
Accepted: July 6, 2016
Published online: September 7, 2016
Processing time: 134 Days and 11.4 Hours
To highlight the potential mechanisms of regeneration in the Associating Liver Partition and Portal vein ligation for Stage hepatectomy models (clinical and experimental) that could unlock the myth behind the extraordinary capability of the liver for regeneration, which would help in designing new therapeutic options for the regenerative drive in difficult setup, such as chronic liver diseases. Associating Liver Partition and Portal vein ligation for Stage hepatectomy has been recently advocated to induce rapid future liver remnant hypertrophy that significantly shortens the time for the second stage hepatectomy. The introduction of Associating Liver Partition and Portal vein ligation for Stage hepatectomy in the surgical armamentarium of therapeutic tools for liver surgeons represented a real breakthrough in the history of liver surgery.
A comprehensive literature review of Associating Liver Partition and Portal vein ligation for Stage hepatectomy and its utility in liver regeneration is performed.
Liver regeneration after Associating Liver Partition and Portal vein ligation for Stage hepatectomy is a combination of portal flow changes and parenchymal transection that generate a systematic response inducing hepatocyte proliferation and remodeling.
Associating Liver Partition and Portal vein ligation for Stage hepatectomy represents a real breakthrough in the history of liver surgery because it offers rapid liver regeneration potential that facilitate resection of liver tumors that were previously though unresectable. The jury is still out though in terms of safety, efficacy and oncological outcomes. As far as Associating Liver Partition and Portal vein ligation for Stage hepatectomy -induced liver regeneration is concerned, further research on the field should focus on the role of non-parenchymal cells in liver regeneration as well as on the effect of Associating Liver Partition and Portal vein ligation for Stage hepatectomy in liver regeneration in the setup of parenchymal liver disease.
Core tip: It seems that liver regeneration after associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) is a combination of portal flow changes and parenchymal transection that generate a systematic response inducing hepatocyte proliferation and remodeling. Further research on the field should focus on the role of non-parenchymal cells as well as on the effect of ALPPS in liver regeneration in the setup of parenchymal liver disease.
- Citation: Moris D, Vernadakis S, Papalampros A, Vailas M, Dimitrokallis N, Petrou A, Dimitroulis D. Mechanistic insights of rapid liver regeneration after associating liver partition and portal vein ligation for stage hepatectomy. World J Gastroenterol 2016; 22(33): 7613-7624
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7613.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7613
Hepatectomy still stands of the first-line treatment modality for malignant liver tumors (primary and metastatic). Postoperative liver failure, though, still consists of the main mortality cause after extended hepatectomy despite the recent advances in surgical techniques due to insufficient future liver remnant (FLR)[1-6].
It is generally agreed that FLR must be around 25% of the liver volume to achieve normal liver function in patients with a healthy liver[1-5]. This leaves only 10% to 20% of patients with primary or metastatic liver disease suitable for surgery at presentation. In patients with drug-induced (chemotherapy) injury or cirrhosis, an FLR of at least 40% is required. In resectable cases, extended hepatectomy offers clear resection margins, that in turn, stands for the major determining factor for long-term survival[1,7,8].
Liver parenchyma is thought to demonstrate unique regenerative capacity but actual mechanisms of the regeneration still remain unclear. More than one strategies have been proposed to induce parenchymal hypertrophy including portal vein embolization (PVE) or ligation (PVL), but the failure rates reach 40% due to tumor progression during the hypertrophic stimulus period (4-8 wk). The regenerative potential associated with these techniques is different from conventional hepatectomy without clear superiority among these techniques (associating PVE or PVL) in terms of hypertrophy of the FLR[9].
Associating liver partition and portal vein ligation for stage hepatectomy (ALPPS) is thought to induce rapid FLR hypertrophy, that in turn, decreases the time for the second stage hepatectomy[10,11]. The introduction of ALPPS in the armamentarium of liver surgeons is, without any doubt, an innovation in liver surgery. It is the last surgical successor of Pychlmayr’s work[12], who first introduced in situ split in liver transplantations. Schnitzbauer et al[10] first described ALPPS, demonstrating an FLR increase of 74% in a short time frame. Unfortunately, the postoperative complication rates[10,13,14] are estimated around 33% to 64% compared with 16% after PVE[15] and much higher that 2-stage hepatectomy[16,17].
ALPPS is getting more familiar in surgical community due to its high variation of indications and modifications[18-20], it still needs further meticulous evaluation before its broader clinical application[21], especially as far as the underlying mechanisms behind the ALPPS-induced accelerated liver regeneration is concerned.
We aim to highlight the potential mechanisms of regeneration in the ALPPS models (clinical and experimental) that could shed some light to the uncharted regenerative capacity of liver parenchyma after ALPPS.
The MEDLINE/PubMed database was searched for publications with the medical subject heading “ALPPS” and keywords “liver regeneration”, “PVL”, and “PVE”. Three independent reviewers (D.M, S.V and A.P) performed the literature search, the study selection and the data extraction. All the references from the identified articles were searched for relevant information. The end date of the literature search was set to April 2016. We focused on articles of any design or scientific method and purpose.
The unique capacity of liver regeneration was described by ancient Greeks who first described the liver regeneration concept in the myth of Prometheus. Having stolen the secret of fire from the gods of Olympus, Prometheus drew down on himself the anger of Zeus, the ruler of gods and men. Zeus punished Prometheus by chaining him to Mount Caucasus where he was tormented by an eagle. The eagle preyed on Prometheus’ liver, which was renewed as fast as it was devoured. An experimental model came to confirm the myth, as rodents undergone two-thirds partial hepatectomy (PHx) demonstrated rapid liver enlargement till the restoration of original liver mass, after which the regenerative process seizes[22].
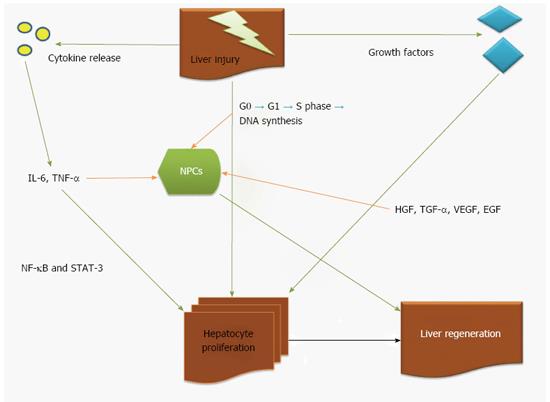
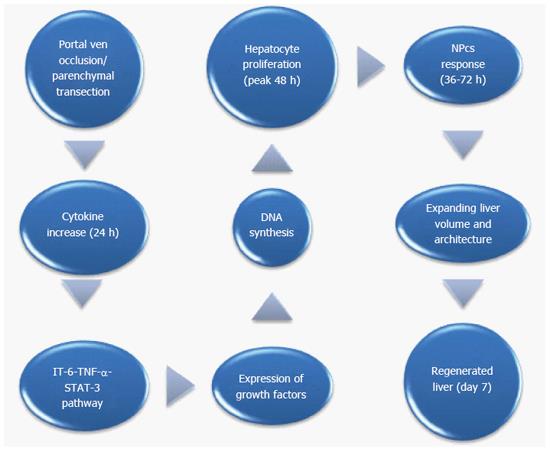
It seems that hypertrophy is based on the proliferation of mature functioning cells in FLR[22-25] and it does not require the recruitment of liver progenitor cells[22-25]. More specifically, adult hepatocytes despite the fact they normally do not undergo cell division, they can proliferate in response to injury[22]. In other words, hepatocytes stay in G0 phase of the cell cycle[23,25] but when a stimulus such as hepatectomy or liver injury occurs, almost all (95%) hepatic cells re-enter the cell cycle inducing DNA synthesis (S phase-12h to 24h). DNA synthesis takes later place in the non-parenchymal cells (NPCs)[22,23,25]. Complete restoration of human liver mass requires less than 2 cycles of replication in all cells[22]. Of interest, the peak in DNA synthesis in rodents occurs later (36 h after PHx)[22]. DNA synthesis begins from periportal area towards the central vein. Most of the increase in liver mass occurs during the first 3 d after PHx and parenchymal restoration is complete in 5-7 d[26].
Hepatocyte proliferation is also induced by potential growth factors as hepatic growth factor (HGF), tumor growth factor-alpha (TGF-α) and the anti-proliferative factor TGF-β[27]. However, it is not well-established if any of these factors play a vital role in liver regeneration itself. HGF induces DNA synthesis in hepatocytes but also alters its morphology. It demonstrates pleiotropic effects on various signaling or downstream pathways, including phosphatidylinositol 3-kinase (PI3K), extracellular signal-regulated kinases (ERK), S6 kinase and AKT[28]. In in vitro setting it was found that the effect of HGF is mediated via up-regulation of TGF-α[29].
Vascular-endothelial growth factor (VEGF) counteracts with liver sinusoids that leads to an increase in HGF production by NPCs. This effect is dependent on endothelial cells with unknown mechanism[30].
Transcription factors including nuclear factor (NF)-κB, signal transducer and activator of transcription (STAT)-3 and AP1[24] are activated in FLR immediately after PHx[22,31] and intracellular-signalling pathways that involve mitogen-activated protein kinase (MAPK) such as pERKs, Jun amino-terminal kinase (JNK) and receptor tyrosine kinases, are also rapidly activated[23,32-34].
NF-κB and STAT-3 transcription factors are activated by cytokines after PHx that led to the consumption that cytokines might regulate the regenerative response[22,31]. Experimental mouse studies demonstrated that after PHx, normal liver regeneration requires IL-6[35,36]. IL-6, though, it was not enough to generate this process, as parenchymal regeneration is only delayed in the absence of IL-6[35].
At the same time, IL-6 is involved in several processes, including hepatoprotection, the acute-phase response and mitogenesis. Binding of IL-6 to its receptor IL-6R, stimulates the tyrosine-kinase activity of the associated Janus-kinase-family (JAK) member[37]. Activated JAK then phosphorylates STAT-3[37]. After PHx, liver regeneration is impaired in IL-6-/- mouse livers with pathognomonic signs that of liver necrosis, reduced hepatocyte-DNA synthesis and discrete G1-phase abnormalities, including decreased STAT-3 activation[35]. The defect is limited to hepatocytes as the DNA-synthesis response seems normal in IL-6-/- NPCs[35].
Similarly, using TNF-receptor-1 (TNF-R1)-/- mice[38], it was found that TNF-α is also compulsory for normal proliferation after PHx via the induction of IL-6. The absence of TNF-α, though, does not affect liver regeneration[39]. Kupffer cells seem to produce most of the IL-6 in the liver[40] and TNF-α induces IL-6 production by enhancing NF-κB, which in turn induced the expression of IL-6.
Figure 1 illustrates all proposed mechanisms of liver regeneration after injury.
As we have already analyzed, after injury, cytokine, growth factors and hormonal expression, induce the beginning and the termination of regeneration[25,41,42]. Parenchymal hypertrophy is also mediated by hemodynamic changes[43-45] and particularly by alterations in portal flow[46]. Portal vein obstruction by PVE or PVL redirects portal vein flow toward specific hepatic segments and is able to pre-operatively increase the volume of FLR. The increase in portal flow to the FLR after PVL or PVE stimulates liver regeneration.
Wilms et al[47] found that recanalization of segmental portal neo-collaterals with occluded portal flow after PVE and PVL are one of the reasons for failure of adequate hypertrophy after technically successful PVE[44,48-50]. Similarly, van Lienden et al[51] investigated intrahepatic vascular changes in patients undergoing PVL and PVE in correlation with hypertrophy and function of the left liver lobe. All patients in PVL group developed intrahepatic portoportal collaterals through which the ligated portal branches are reperfused within 3 wk and one patient (7.1%) in the PVE group[51]. The median increase of FRL volume after PVE was 41.6 % (range: 10%-305%), and after PVL was only 8.1% (range 0%-102%) (P = 0.179). There were no differences in FRL function between both groups[51]. The absence of collaterals and recanalization may explain the greater hypertrophy found in ALPPS, an issue with very high clinical impact[48-50].
ALPPS succeeds in preventing neo-collateral formation across the ligated and non-ligated liver lobes with additional hepatic parenchyma transaction step. Parenchymal transection in stage I ALPPS also creates a traumatic stimulus, which may also contribute to the hypertrophy[49]. PVL/PVE alone often fail to generate adequate hypertrophy because of distal porto-portal collateral formation[52] and proximal portal vein occlusion causes portal cavernoma and recirculation of blood from the non-occluded liver to the occluded one[53]. Dhar et al[54] demonstrated portal pressure increase in the ALPPS group compared with the ligation group, that in turn enhances shear stress and provokes early regeneration in the ALPPS group. Shindoh et al[55] demonstrated a variant of PVE involving the segment IV that achieves similar to ALPPS parenchymal regeneration. PVE though is superior in terms of FLR regeneration compared with liver resection alone[56].
The Ki-67 protein is a well-established cellular marker for proliferation that is present in active cells, but is absent in resting cells (G0)[57]. In ALPPS setting (experimental and clinical), the increase of Ki-67 expression after the procedure is a common finding.
In experimental setting, Schlegel et al[42] found a rapid liver parenchymal increase (100% in the first 24h) after stage I ALPPS that was combined with a significantly higher Ki-67 expression compared with simple transection, PVL and PHx group[42]. These results indicate that increased number of hepatocytes enter the cell cycle after ALPPS than any other intervention[42].
Furthermore, Dhar et al[54] demonstrated a significant FLR increase combined with increased periportal hepatocyte proliferation (Ki-67 index) in the ALPPS group compared with the PVL group at 24 h (P = 0.002) and 48 h (P = 0.031)[54].
In the same frame, Wei et al[58] evaluated the proliferation (1:50 monoclonal anti-BrdU antibody) of hepatocytes after ALPPS compared with non-ALPPS group (PVL, simple transection, PHx). The 2-fold increase of liver lobe weight was combined with an increased proliferation index (15.4% ± 0.9%) in ALPPS group compared to control (8.6% ± 2.9%, P = 0.009)[58].
García-Pérez et al[59] demonstrated in an experimental model of ALPPS that mitotic figures detected at 48 h were more prominent in ALPPS FLR compared with PVL (P < 0.0001). As far as proliferation potential is concerned, higher expression of Ki-67 was related with in the ALPPS group at 48 h (P < 0.001 compared with PVL group)[59]. Finally, Shi et al[60] demonstrated in an experimental model that ALPPS was correlated with increased liver regeneration (liver weight to body weight ratio) and increased hepatocyte proliferation assessed by Ki-67 and proliferating cell nuclear antigen (PCNA) activity compared with PHx and PVL group.
In pediatric liver with hepatoblastoma, a rapid increase of FLR (46.1%) after stage I ALPPS was noted with concomitant increased expression of Ki-67 in the left liver (proliferation index of right liver and left lateral segment-LLS to be 2% and 20% respectively)[61].
The proliferation after PVL can also affect tumor cells in the affected hemiliver. Kokudo et al[62] found increased Ki-67 expression of intrahepatic metastases in the embolized liver after PVE. This finding is similar in ALPPS despite a shorter interval between PVL and parenchymal resection. In small series, an increase in Ki-67 expression from 60% at stage I ALPPS to 80% at stage II was noted[49,63].
More specifically, Tanaka et al[49] evaluated the proliferation of tumor cells after step I ALPPS in patients with unresectable multiple liver metastases from colorectal cancer (CLM) and pancreatic neuroendocrine tumor. Control group for the comparison was chosen a group of patients with CLM initially considered unresectable with classical 2-stage hepatectomy. They demonstrated an increase of FLR in ALPPS group of 40%-50% which was less than the one observed in the classical 2-stage hepatectomy group (P < 0.01)[49], probably due to relatively large volume of liver parenchyma heavily pretreated by chemotherapy in ALPPS group, resulting in less capacity for regeneration. Additionally, Ki67 expression in tumor cells was lower in ALPPS group compared to classical 2-stage group (P = 0.09)[49]. This finding may support a potential oncologic benefit from ALPPS, with the short period between the 2 interventions helping to avoid risk of tumor progression.
Similarly, Matsuo et al[64] evaluated the proliferation potential in liver parenchyma after ALPPS or 2-stage hepatectomy in patients with colonic liver metastases. They used for this purpose a monoclonal antibody against the Ki-67 antigen (MIB-1, 1:100). The mean increase of FLR after stage I ALPPS was 50% with concomitant increased MIB-1 labeling (expressed in 7.8% ± 4.9% of hepatocytes in the ALPPS group, compared to PVE group, 0.9% ± 0.7%, P = 0.01)[64].
In ALPPS setting, both TNF-α and HGF can activate JNK and MAPK-ERK pathways and they can also induce the expression of cyclin D1[65]. In the regenerating liver, activation of cyclin D1 induces the progression of cell cycle through G1 and entry into S phase[66]. Cyclin-D1 expression is induced by IL-6 whereas the expression of the S-phase cyclins A and B1 is induced by insulin-like-growth-factor binding protein[22].
Shi et al[60] evaluated the effect of the different procedures (ALPPS, PVL and transection) on cell cycle regulators in experimental setting. Data extracted from immunochemical staining indicated that ALPPS stimulated cyclin D1 and cyclin E expression more significantly compared with other procedures and the maximum induction occurred on day 3 and day 2, respectively (P < 0.01 and 0.001 respectively)[60]. G1 Cdks as catalytic partner of cyclin E and cyclin D1 to facilitate cells entering S phase. They were also induced at 24 h after ALPPS, with a maximum induction on day 3 compared with sham group[60]. Cdk2-associated kinase activity was also increased during liver regeneration after ALPPS[60]. Collectively, these results demonstrated not only temporal increases in the cyclin E/Cdk2 complex, but also concomitant increases in Cdk2 kinase activity during hepatocyte proliferation after ALPPS.
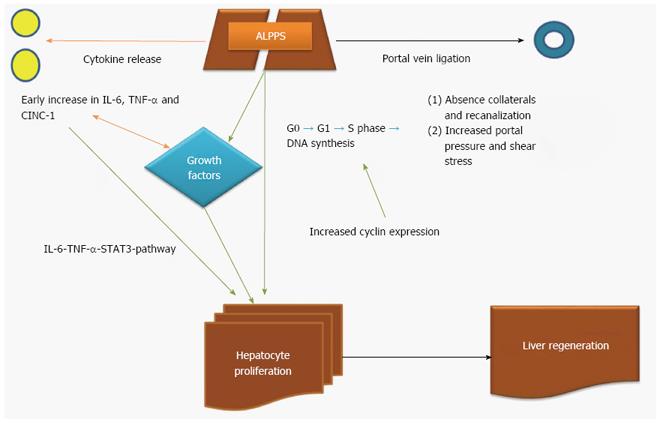
In the experimental setting, Schlegel et al[42] demonstrated that ALPPS-plasma injection after PVL triggers comparable regeneration in terms of liver weight gain and regeneration, as original ALPPS which was not the case when plasma obtained from group of sham laparotomy did not present any additional regeneration when injected in PVL-treated livers. On the basis of these experiments, it seems that the rapid liver volume increase after ALPPS is triggered by systemic release of putative initiators. After meticulous analysis of the ALPPS plasma in PVL-treated mice, an early increase of plasma IL-6 levels compared to PVL alone was found[42]. Of interest though, transection alone induced similarly increased IL-6-expression at this early time point[42]. Genomic evaluation revealed significant up-regulation of IL-6-mRNA and TNF-α-mRNA 1 h after step I of ALPPS or transection. These findings were confirmed in clinical setting, where increased gene expression of IL-6 and TNF-α was found in liver tissue and plasma 1 hour after step I ALPPS or PVL alone[42]. All in all, the contribution of the IL-6-TNF-α-STAT3-pathway to the rapid liver hypertrophy after step I ALPPS is suggested in both clinical and experimental setting. Moreover, no changes in CD31 and VEGF expression was found in regenerating lobe during the first week after ALPPS or PVL[42].
In the same setting, Dhar et al[54] demonstrated that the cytokine-induced neutrophil chemoattractant -1 (CINC-1) had its highest levels in liver tissue with main difference in expression between the ALPPS and PVL group was in IL-6 expression at 24 h[54]. Finally, ALPPS group demonstrated increased VEGF and interferon gamma expression at 48 h[54]. In terms of inflammatory cells, ALPPS group demonstrated higher early infiltration of liver by inflammatory cells compared with PVL (P = 0.021)[54].
Shi et al[60] also investigated gene and cytokine involvement in liver regeneration after ALPPS. Results indicated that the levels of IL-6, NF-κB p65, STAT3, TNF-α, EGF, HGF, ERK-1/2 and YAP were significantly increased in the ALPPS group compared with the other groups (PVL and transection alone), suggesting that these factors might play important roles in the fast liver regeneration after ALPPS[60]. As expected, the mRNA levels of these cytokines were also highly increased in the regenerating lobes 24 h, 3 d and 5 d after ALPPS[60].
García-Pérez et al[59] showed in an experimental model of ALPPS that HGF expression in FLR after PVL was increased compared with ALPPS at 24 h (P < 0.05)[59]. At 48h, ALPPS was associated with higher expression of HGF and TGF-α (P < 0.05) with similar relative expression of TGF-β in both groups[59]. Later, the HGF and TGF-β levels were still higher on ALPPS group (P < 0.005 and P < 0.05 respectively)[59]. Interestingly, hepatic transection caused an increase in the number of Kupffer cells in ALPPS group at 24 h (P < 0.01)[59].
Table 1 summarizes the data of the experimental models of ALPPS. Table 2 summarizes the mechanistic effect of each modality (PVE, PVL, simple transection and ALPPS) in liver regeneration. Figures 2 and 3 illustrate the potential mechanisms of liver regeneration after ALPPS as well as the cascade of events leading to liver volume restoration.
| Ref. | Year | Species | PVL | PHx in stage I | PHx in stage II | Loss of liver mass | Atrophy of ligated lobe on day 7 | Future remnant liver lobe | Fold increase on day 7 | Proliferation |
| Schlegel et al[42] | 2014 | Mouse | RML | LLL (30%) | Ligated lobes | 85% totally (55% PVL + 30% PHx) | NA | LML (15%) | 4-fold | Peaked on day 4 |
| RL | ||||||||||
| CL | ||||||||||
| Yao et al[70] | 2014 | Rat | LLL | NA | NA | 80% PVL | NA | RML (20%) | 2.5-fold | Peaked on day 2 |
| LML | ||||||||||
| RL | ||||||||||
| CL | ||||||||||
| Almau Trenard et al[71] | 2014 | Rat | LLL | NA | NA | 80% PVL | Reduction to 35.2% | RML (20%) | 2-fold | NA |
| LML | ||||||||||
| RL | ||||||||||
| CL | ||||||||||
| Dhar et al[54] | 2015 | Rat | LLL | NA | NA | 80% PVL | NA | RML (20%) | 2-fold | Peaked on day 2 |
| LML | ||||||||||
| RL | ||||||||||
| CL | ||||||||||
| Wei et al[58] | 2015 | Rat | LLL | CL (10%) | Ligated lobes | 80% totally (70% PVL + 10% PHx) | Reduction to 48.2% | RML (20%) | 2.53-fold | Peaked on day 1 |
| LML | ||||||||||
| RL | ||||||||||
| García-Pérez et al[59] | 2015 | Rat | RSL | RML (20%) | Ligated lobes | 80% totally (60% PVL + 20% PHx) | N/A | LML (22%) | 2-fold | Peaked on day 2 |
| RIL | ||||||||||
| LLL | ||||||||||
| RML | ||||||||||
| Shi et al[60] | 2015 | Rat | RML | LLL (30%) | Ligated lobes | 80% totally (50% PVL + 30% PHx) | Reduction to 34.9% | LML (20%) | 2.3 fold | Peaked on day 2 |
| RL | ||||||||||
| CL |
| Intervention | Cytokine release | Growth factor expression | DNA synthesis | Portal vein flow decrease | Hepatocyte hypertrophy induction | Increased NPC activity | Atrophy of the affected lobe | Ref. |
| PVL | ++ | ++ | ++ | ++ | ++ | ++ | ++ | [42,54,58-60,70,71] |
| PVE | + | + | + | + | + | ++ | + | [42,54, 58-60,70,71] |
| Parenchymal Transection | +++ | + | + | 0 | + | + | + | [42,47-49,54,58-61,64,70-72] |
| ALPPS | +++ | +++ | +++ | +++ | +++ | + | +++ | [42,47-49,54,58-61,64,70-72] |
Matsuo et al[64] evaluated the special histologic characteristics of hepatocytes after ALPPS compared with liver tissue retrieved from patients undergoing PVE or staged hepatectomy PVE after chemotherapy. In the area of the FLR, glycogen-rich, vacant-cytoplasm bright-appearing hepatocytes were more frequent in ALPPS than in PVE. Both hepatocyte brightness and sinusoidal narrowing were observed more frequently in ALPPS than in PVE (P = 0.025)[64]. Hepatocyte cell density was greater and hepatocyte size was smaller in the ALPPS group than in the PVE group (P < 0.01)[64]. In the ligated liver part, hepatocyte atrophy, degeneration or necrosis, sinusoidal dilation, fibrosis and congestion all were more frequent with ALPPS than with PVE (P = 0.001, P = 0.001, P = 0.002, P < 0.001, and P < 0.001, respectively)[64]. Cytoplasmic organelles such as mitochondria and endoplasmic reticulum were fewer than in the PVE group[64]. All these findings are indicative of cell immaturity in ALPPS setting.
In an experimental model of ALPPS, García-Pérez et al[59] demonstrated that the main histological features on the atrophic lobes were periportal congestion, sinusoid dilation and areas of necrotic or apoptotic hepatocytes.
The ALPPS technique has taken its place in liver surgery as alternative in those cases in which the FLR volume is inadequate for normal liver after parenchymal resection and shortens the gap between the first and second step avoiding the risk of tumor progression. This rising interest in ALPPS has, and will further, evaluate the efficacy of technical innovations to address the initial concern about high complication rates and long-term survival[67].
This analysis shed light to the potential mechanisms involved to liver regeneration after ALPPS by presenting well-established knowledge on liver regeneration per se and emerging knowledge on liver regeneration induction after ALPPS, PVL, PVE and simple parenchymal transection.
In a meta-analysis of portal vein obstruction as a stimulus to induce liver hypertophy, PVE with various methods of embolization induced a mean volumetric increment by 8%-27% over a period of 2-6 wk[15] and PVL achieved a FLR volumetric increment by 38%-53% over a period of 4-8 wk[68]. These differences in liver regeneration indicate the crucial role of portal vein occlusion in inducing the regeneration process. On the contrary, ALPPS procedure achieves a variable increase in the FLR ranging from 21%-200%. This could be partially attributable to the presence of underlying parenchymal liver diseases (cirrhosis and chronic liver diseases).
At the same time, besides enhancing extended liver hypertrophy, ALPPS procedure increases liver atrophy compared with PVL without transection. This could be mainly attributed to the inadequate portal blood supply that causes significant atrophy that stands for greater liver mass and volume loss, which stands of an important regenerative trigger[69].
Novel knowledge about the mechanisms of liver regeneration after ALPPS were established by many variants of ALPPS model. Seven different experiments of ALPPS were reported in the literature[9,42,70,71]. These models included the transection of the median lobe, but differed on the extent of the FLR, PVL the additional PHx. The mouse model of Schlegel et al[42] demonstrated a 55% PVL and a 30% PHx in the first stage ALPPS. The rat models[54,58-60,70,71] consisted of an 80% PVL via ligation of all but the right median lobe. Mouse model demonstrated a regenerative potential on the first postoperative day and reached the peak on postoperative day 4[22]. Yao et al[70], García-Pérez et al[59] and Dhar et al[54] observed a proliferation peak on day 2, which is usually observed after classic PVL. Despite the fact that liver mass reduction is similar to all models, Wei et al[58] demonstrated a peak of proliferation on first postoperative day, as observed after the classic 70% PHx. Subsequently, the increase in FLR in that study was slightly higher than other rat models. The differences in proliferative kinetics suggest that even small differences in the ratio between the extent of PVL and PHx may produce a substantial effect on the time course of intrahepatic size regulation. The main disadvantage of these models is that they evaluated liver regeneration after injury in healthy tissues, which is not representative to clinical practice. Future research should focus on liver regeneration after ALPPS in models of liver disease.
At the same time, several clinical settings evaluating the regenerative potential after ALPPS in patients with primary or secondary (colonic) metastatic disease[48,49,61,64,72]. The design of these studies included a group of patients with metastatic liver disease and a group of patients with unresectable tumors that underwent 2-stage hepatectomy. The common finding of these studies was that the ALPPS group demonstrated a higher hepatocyte proliferation potential (assessed by Ki-67).
As far as the mechanistics of liver regeneration after ALPPS is concerned, Schlegel et al[42] showed that parenchymal transection induces an inflammatory response in terms of growth factor and cytokines release contributing to regeneration. The response, though, is not organ-specific, induced by soluble initiators in the circulation. The observation of accelerated regeneration, when injecting plasma obtained from mice after step I ALPPS to animals undergoing PVL alone, strongly supports the existence of soluble growth factors. The similar effects on regeneration achieved by injuries to other organs further point out to existence of soluble mediators of liver regeneration, additionally suggesting that the origin of the circulating growth factors is not “liver-specific.”
The detection of similar enhanced release of proinflammatory cytokines in samples obtained in ALPPS and PVL[42] patients is indicative of the crucial role of portal vein flow changes in inducing the regeneration process, especially early postoperatively. After the initial stage of injury due to portal vein flow changes, ALPPS group demonstrated dramatic increase of instigators of regeneration (IL-6 and TNF-α) compared with PVL alone but similar to transection alone[42]. Surgical procedures of similar invasiveness (split liver in liver transplantation or living liver donation) were shown to induce comparable systemic inflammation and cytokine release as in the liver tissue early after step 1 of ALPPS[24,73,74]. This finding is indicative that ALPPS due to its dual nature (PVL+ transection) manages to keep high levels of expression of cytokines and growth factors that could explain the rapid liver regeneration potential of this technique. Liver transection with the PVL can induced programmed cell death followed by cytokine surge that facilitates accelerated liver regeneration.
Hepatocytes are the crucial player in liver regeneration after ALPPS since liver injury initiates high expression of cytokines, growth factors and cell cycle promoters, such as cyclins that lead quiescent hepatocytes to enter G1 and S phase of cell cycle and eventually proliferate (assessed by Ki-67 expression). This concept is similar to liver regeneration after injury of any kind. The leading pathway of the cascade above is IL-6- TNF-α-STAT-3 pathway. One interesting finding though is that after ALPPS, the majority of the hepatocytes that contribute to the regeneration are immature cells (narrowing of sinusoidal spaces, organelle distribution pattern) compared to the mature cells (numerous lipofuscin granules) that are involved in regeneration process after PVE[64,75]. Proliferation of hepatocytes begins from periportal area and is directed towards central vein area.
Among the wide range of cytokines examined in the literature, Dhar et al[54] suggested that CINC-1 had the strongest and early significant expression in the ALPPS liver when compared with the PVL liver, supporting a crucial role of CINC-1 in the ALPPS associated regeneration. Interestingly, Kaibori et al[76] noticed that CINC-1 induced by HGF produced enhanced proliferative and angiogenic activities through NFkB activation in the liver. The increased IL-6 level concords with the majority of animal models of ALPPS, where a significant increase in plasma IL-6 in the ALPPS group compared with the PVL group is demonstrated. Early activation of IL-6/STAT3 pathway in macrophages facilitates the generation of chemokines such as MIP-a and the migration of bone marrow derived MSCs to the liver during the restitution of liver mass[77].
The role of NPCs (Kupffer cells, endothelial cells) in liver regeneration after ALPPS is not elucidated. The majority of studies agree that its role is supportive rather than fundamental in terms of maintaining a cytokine-rich liver microenvironment (INF-γ, IL-6) that induces local inflammatory response to ALPPS. In different setting, Tanaka et al[78] found an increased number of Kupffer cells infiltration following PVE + PHx compared with PHx only group following endotoxin challenge in rats. Literature stands also equivocal towards VEGF, but it seems that throughout this regenerative process, not extensive angiogenesis takes place. All in all, liver parenchymal cells, as well as resident NPCs and infiltrated inflammatory cells seem to play crucial roles in the ALPPS-induced liver hypertrophy.
ALPPS represents a real breakthrough in the history of liver surgery because it offers rapid liver regeneration potential that facilitate resection of liver tumors that were previously though unresectable. The jury is still out though in terms of safety, efficacy and oncological outcomes. Liver regeneration after ALPPS is a combination of portal flow changes and parenchymal transection that generate a systematic response inducing hepatocyte proliferation and remodeling. Further research on the field should focus on the role of NPCs in liver regeneration as well as on the effect of ALPPS in liver regeneration in the setup of parenchymal liver disease.
Associating liver partition and portal vein ligation for stage hepatectomy (ALPPS) has been recently advocated to induce rapid future liver remnant hypertrophy that significantly shortens the time for the second stage hepatectomy
Liver regeneration after ALPPS is a combination of portal flow changes and parenchymal transection that generate a systematic response inducing hepatocyte proliferation and remodeling. To date, no data are questions are risen about the safety, efficacy and oncological outcomes of ALPPS. In terms of liver regeneration, further research on the field should focus on the role of non-parenchymal cells in liver regeneration as well as on the effect of ALPPS in liver regeneration in the setup of parenchymal liver disease.
This is-the first to the authors knowledge-systematic review about the mechanisms of liver regeneration after ALPPS.
This article highlights the potential mechanisms of regeneration in the ALPPS models (clinical and experimental) that could unlock the myth behind the extraordinary capability of the liver for regeneration, which would help in designing new therapeutic options for the regenerative drive in difficult setup, such as chronic liver diseases.
ALPPS operation is divided in two steps. The first consists of exploratory laparotomy, assessment of resectability with intraoperative ultrasound and positioning the tumor in relation with vessels. The liver is mobilized by dissecting the ligaments. The right liver lobe is completely mobilized from the cava vein. After the right portal vein branch is identified, it is divided. Right hepatic artery and right hepatic duct are identified and also kept. Finally, total parenchymal dissection at the right of the falciform ligament is performed. After in situ splitting, the right lobe is covered by a biomaterial and the abdomen is drained and closed. The second step of the procedure is completed by re-laparotomy. The right hepatic artery, right hepatic duct and the right hepatic vein are ligated. The liver resection is completed. The left lateral lobe is then fixed to the remnant falciform ligament.
The manuscript provides a wide vision of liver regeneration in the context of different therapeutic interventions combining results from experimental models and from the clinical management of patients suffering from parenchymal liver disorders. All issues discussed are relevant, provide molecular mechanisms that might well explain the clinical observations and leave still open questions that will surely foster future investigation avenues.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Corrales FJ, Shi YJ S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Björnsson B, Sparrelid E, Røsok B, Pomianowska E, Hasselgren K, Gasslander T, Bjørnbeth BA, Isaksson B, Sandström P. Associating liver partition and portal vein ligation for staged hepatectomy in patients with colorectal liver metastases--Intermediate oncological results. Eur J Surg Oncol. 2016;42:531-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-1049; discussion 1049-1051. [PubMed] [Cited in This Article: ] |
| 3. | Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829-836; discussion 836-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 333] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 4. | Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, Baumgart J, Croome K, Hernandez-Alejandro R, Lang H. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 5. | Tschuor Ch, Croome KP, Sergeant G, Cano V, Schadde E, Ardiles V, Slankamenac K, Clariá RS, de Santibaňes E, Hernandez-Alejandro R, Clavien PA. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the ALPPS approach. Eur J Surg Oncol. 2013;39:1230-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Zhang GQ, Zhang ZW, Lau WY, Chen XP. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new strategy to increase resectability in liver surgery. Int J Surg. 2014;12:437-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347-353. [PubMed] [Cited in This Article: ] |
| 8. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Makuuchi M. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219-1222. [PubMed] [Cited in This Article: ] |
| 9. | Gock M, Eipel C, Linnebacher M, Klar E, Vollmar B. Impact of portal branch ligation on tissue regeneration, microcirculatory response and microarchitecture in portal blood-deprived and undeprived liver tissue. Microvasc Res. 2011;81:274-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 930] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 11. | de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255:415-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 12. | Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988;373:127-130. [PubMed] [Cited in This Article: ] |
| 13. | Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro R, Jovine E, Machado M. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg. 2015;262:780-785; discussion 785-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien PA. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol. 2015;22:3109-3120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 16. | Ratti F, Schadde E, Masetti M, Massani M, Zanello M, Serenari M, Cipriani F, Bonariol L, Bassi N, Aldrighetti L. Strategies to Increase the Resectability of Patients with Colorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann Surg Oncol. 2015;22:1933-1942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Bertens KA, Hawel J, Lung K, Buac S, Pineda-Solis K, Hernandez-Alejandro R. ALPPS: challenging the concept of unresectability--a systematic review. Int J Surg. 2015;13:280-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Fard-Aghaie MH, Stavrou GA, Schuetze KC, Papalampros A, Donati M, Oldhafer KJ. ALPPS and simultaneous right hemicolectomy - step one and resection of the primary colon cancer. World J Surg Oncol. 2015;13:124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Petrou A, Moris D, Kountourakis P, Fard-Aghaie M, Neofytou K, Felekouras E, Papalampros A. The ALPPS procedure as a novel “liver-first” approach in treating liver metastases of colon cancer: the first experience in Greek Cypriot area. World J Surg Oncol. 2016;14:67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Chan AC, Poon RT, Chan C, Lo CM. Safety of ALPPS Procedure by the Anterior Approach for Hepatocellular Carcinoma. Ann Surg. 2016;263:e14-e16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Moris D, Dimitroulis D, Papalampros A, Petrou A, Felekouras E. ALPPS Procedure for Hepatocellular Carcinoma in Patients With Chronic Liver Disease: Revealing a Terra Incognita. Ann Surg. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1091] [Cited by in F6Publishing: 1139] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 24. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1067] [Cited by in F6Publishing: 1112] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 25. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [PubMed] [Cited in This Article: ] |
| 26. | Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962;22:842-849. [PubMed] [Cited in This Article: ] |
| 27. | Moolten FL, Bucher NL. Regeneration of rat liver: transfer of humoral agent by cross circulation. Science. 1967;158:272-274. [PubMed] [Cited in This Article: ] |
| 28. | Okano J, Shiota G, Matsumoto K, Yasui S, Kurimasa A, Hisatome I, Steinberg P, Murawaki Y. Hepatocyte growth factor exerts a proliferative effect on oval cells through the PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2003;309:298-304. [PubMed] [Cited in This Article: ] |
| 29. | Tomiya T, Ogata I, Yamaoka M, Yanase M, Inoue Y, Fujiwara K. The mitogenic activity of hepatocyte growth factor on rat hepatocytes is dependent upon endogenous transforming growth factor-alpha. Am J Pathol. 2000;157:1693-1701. [PubMed] [Cited in This Article: ] |
| 30. | LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 530] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 31. | Taub R, Greenbaum LE, Peng Y. Transcriptional regulatory signals define cytokine-dependent and -independent pathways in liver regeneration. Semin Liver Dis. 1999;19:117-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Haber BA, Mohn KL, Diamond RH, Taub R. Induction patterns of 70 genes during nine days after hepatectomy define the temporal course of liver regeneration. J Clin Invest. 1993;91:1319-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 152] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Kelley-Loughnane N, Sabla GE, Ley-Ebert C, Aronow BJ, Bezerra JA. Independent and overlapping transcriptional activation during liver development and regeneration in mice. Hepatology. 2002;35:525-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Behrens A, Sibilia M, David JP, Möhle-Steinlein U, Tronche F, Schütz G, Wagner EF. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J. 2002;21:1782-1790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [PubMed] [Cited in This Article: ] |
| 36. | Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, Demetris AJ. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 215] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2296] [Cited by in F6Publishing: 2333] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 38. | Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441-1446. [PubMed] [Cited in This Article: ] |
| 39. | Fujita J, Marino MW, Wada H, Jungbluth AA, Mackrell PJ, Rivadeneira DE, Stapleton PP, Daly JM. Effect of TNF gene depletion on liver regeneration after partial hepatectomy in mice. Surgery. 2001;129:48-54. [PubMed] [Cited in This Article: ] |
| 40. | Aldeguer X, Debonera F, Shaked A, Krasinkas AM, Gelman AE, Que X, Zamir GA, Hiroyasu S, Kovalovich KK, Taub R. Interleukin-6 from intrahepatic cells of bone marrow origin is required for normal murine liver regeneration. Hepatology. 2002;35:40-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Mortensen KE, Revhaug A. Liver regeneration in surgical animal models - a historical perspective and clinical implications. Eur Surg Res. 2011;46:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Schlegel A, Lesurtel M, Melloul E, Limani P, Tschuor C, Graf R, Humar B, Clavien PA. ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg. 2014;260:839-46; discussion 846-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 43. | Riehle KJ, Dan YY, Campbell JS, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;26 Suppl 1:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 44. | Abshagen K, Eipel C, Vollmar B. A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch Surg. 2012;397:579-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg. 2007;31:367-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Mortensen KE, Conley LN, Nygaard I, Sorenesen P, Mortensen E, Bendixen C, Revhaug A. Increased sinusoidal flow is not the primary stimulus to liver regeneration. Comp Hepatol. 2010;9:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Wilms C, Mueller L, Lenk C, Wittkugel O, Helmke K, Krupski-Berdien G, Rogiers X, Broering DC. Comparative study of portal vein embolization versus portal vein ligation for induction of hypertrophy of the future liver remnant using a mini-pig model. Ann Surg. 2008;247:825-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Tanaka K, Endo I. ALPPS: Short-term Outcome and Functional Changes in the Future Liver Remnant. Ann Surg. 2015;262:e88-e89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Tanaka K, Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Endo I, Ichikawa Y, Taguri M, Tanabe M. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur J Surg Oncol. 2015;41:506-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Gall TM, Sodergren MH, Frampton AE, Fan R, Spalding DR, Habib NA, Pai M, Jackson JE, Tait P, Jiao LR. Radio-frequency-assisted Liver Partition with Portal vein ligation (RALPP) for liver regeneration. Ann Surg. 2015;261:e45-e46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | van Lienden KP, Hoekstra LT, Bennink RJ, van Gulik TM. Intrahepatic left to right portoportal venous collateral vascular formation in patients undergoing right portal vein ligation. Cardiovasc Intervent Radiol. 2013;36:1572-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Denys AL, Abehsera M, Sauvanet A, Sibert A, Belghiti J, Menu Y. Failure of right portal vein ligation to induce left lobe hypertrophy due to intrahepatic portoportal collaterals: successful treatment with portal vein embolization. AJR Am J Roentgenol. 1999;173:633-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Yamakado K, Takeda K, Nishide Y, Jin J, Matsumura K, Nakatsuka A, Hirano T, Kato N, Nakagawa T. Portal vein embolization with steel coils and absolute ethanol: a comparative experimental study with canine liver. Hepatology. 1995;22:1812-1818. [PubMed] [Cited in This Article: ] |
| 54. | Dhar DK, Mohammad GH, Vyas S, Broering DC, Malago M. A novel rat model of liver regeneration: possible role of cytokine induced neutrophil chemoattractant-1 in augmented liver regeneration. Ann Surg Innov Res. 2015;9:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A, Gupta S, Wallace MJ, Aloia TA. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217:126-133; discussion 133-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 56. | Meyer IA, Vandoni RE, Alerci M, Raptis DA, Gertsch P. Portal Vein Embolization Followed by Liver Resection versus Liver Resection Alone: a Comparison of Liver Regeneration Dynamics. Hepatogastroenterology. 2015;62:987-991. [PubMed] [Cited in This Article: ] |
| 57. | Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 58. | Wei W, Zhang T, Zafarnia S, Schenk A, Xie C, Kan C, Dirsch O, Settmacher U, Dahmen U. Establishment of a rat model: Associating liver partition with portal vein ligation for staged hepatectomy. Surgery. 2016;159:1299-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | García-Pérez R, Revilla-Nuin B, Martínez CM, Bernabé-García A, Baroja Mazo A, Parrilla Paricio P. Associated Liver Partition and Portal Vein Ligation (ALPPS) vs Selective Portal Vein Ligation (PVL) for Staged Hepatectomy in a Rat Model. Similar Regenerative Response? PLoS One. 2015;10:e0144096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Shi H, Yang G, Zheng T, Wang J, Li L, Liang Y, Xie C, Yin D, Sun B, Sun J. A preliminary study of ALPPS procedure in a rat model. Sci Rep. 2015;5:17567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Chan A, Chung PH, Poon RT. Little girl who conquered the “ALPPS’’. World J Gastroenterol. 2014;20:10208-10211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 278] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 63. | Fukami Y, Kurumiya Y, Kobayashi S. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): an analysis of tumor activity. Updates Surg. 2014;66:223-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Yamazaki K, Ishida Y, Tanaka K. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery. 2016;159:1289-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 65. | Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Nelsen CJ, Rickheim DG, Tucker MM, Hansen LK, Albrecht JH. Evidence that cyclin D1 mediates both growth and proliferation downstream of TOR in hepatocytes. J Biol Chem. 2003;278:3656-3663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Clavien PA, Lillemoe KD. Note from the editors on the ALPPS e-Letters-to-the-Editor. Ann Surg. 2012;256:552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Kianmanesh R, Farges O, Abdalla EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003;197:164-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Niehues SM, Unger JK, Malinowski M, Neymeyer J, Hamm B, Stockmann M. Liver volume measurement: reason of the difference between in vivo CT-volumetry and intraoperative ex vivo determination and how to cope it. Eur J Med Res. 2010;15:345-350. [PubMed] [Cited in This Article: ] |
| 70. | Yao L, Li C, Ge X, Wang H, Xu K, Zhang A, Dong J. Establishment of a rat model of portal vein ligation combined with in situ splitting. PLoS One. 2014;9:e105511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Almau Trenard HM, Moulin LE, Padín JM, Stringa P, Gondolesi GE, Barros Schelotto P. Development of an experimental model of portal vein ligation associated with parenchymal transection (ALPPS) in rats. Cir Esp. 2014;92:676-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Røsok BI, Björnsson B, Sparrelid E, Hasselgren K, Pomianowska E, Gasslander T, Bjørnbeth BA, Isaksson B, Sandström P. Scandinavian multicenter study on the safety and feasibility of the associating liver partition and portal vein ligation for staged hepatectomy procedure. Surgery. 2016;159:1279-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | von Heesen M, Hülser M, Seibert K, Scheuer C, Dold S, Kollmar O, Wagner M, Menger MD, Schilling MK, Moussavian MR. Split-liver procedure and inflammatory response: improvement by pharmacological preconditioning. J Surg Res. 2011;168:e125-e135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3:485-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 75. | Miyaoka Y, Miyajima A. To divide or not to divide: revisiting liver regeneration. Cell Div. 2013;8:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | Kaibori M, Yanagida H, Nakanishi H, Ozaki T, Yoshida H, Matsui K, Hijikawa T, Kamiyama Y, Okumura T. Hepatocyte growth factor stimulates the induction of cytokine-induced neutrophil chemoattractant through the activation of NF-kappaB in rat hepatocytes. J Surg Res. 2006;130:88-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2013;288:1489-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 78. | Tanaka H, Kinoshita H, Hirohashi K, Kubo S, Lee KC. Increased safety by two-stage hepatectomy with preoperative portal vein embolization in rats. J Surg Res. 1994;57:687-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |