Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.7157
Peer-review started: April 14, 2016
First decision: May 12, 2016
Revised: May 26, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: August 21, 2016
Processing time: 124 Days and 0.6 Hours
AIM: To compare disease-free survival (DFS) between extramural vascular invasion (EMVI)-positive and -negative colon cancer patients evaluated by computed tomography (CT).
METHODS: Colon cancer patients (n = 194) undergoing curative surgery between January 2009 and December 2013 were included. Each patient’s demographics, cancer characteristics, EMVI status, pathological status and survival outcomes were recorded. All included patients had been routinely monitored until December 2015. EMVI was defined as tumor tissue within adjacent vessels beyond the colon wall as seen on enhanced CT. Disease recurrence was defined as metachronous metastases, local recurrence, or death due to colon cancer. Kaplan-Meier analyses were used to compare DFS between the EMVI-positive and -negative groups. Cox’s proportional hazards models were used to measure the impact of confounding variables on survival rates.
RESULTS: EMVI was observed on CT (ctEMVI) in 60 patients (30.9%, 60/194). One year after surgery, there was no statistically significant difference regarding the rates of progressive events between EMVI-positive and -negative patients [11.7% (7/60) and 6.7% (9/134), respectively; P = 0.266]. At the study endpoint, the EMVI-positive patients had significantly more progressive events than the EMVI-negative patients [43.3% (26/60) and 14.9% (20/134), respectively; odds ratio = 4.4, P < 0.001]. Based on the Kaplan-Meier method, the cumulative 1-year DFS rates were 86.7% (95%CI: 82.3-91.1) and 92.4% (95%CI: 90.1-94.7) for EMVI-positive and EMVI-negative patients, respectively. The cumulative 3-year DFS rates were 49.5% (95%CI: 42.1-56.9) and 85.8% (95%CI: 82.6-89.0), respectively. Cox proportional hazards regression analysis revealed that ctEMVI was an independent predictor of DFS with a hazard ratio of 2.15 (95%CI: 1.12-4.14, P = 0.023).
CONCLUSION: ctEMVI may be helpful when evaluating disease progression in colon cancer patients.
Core tip: The 4-point computed tomography extramural vascular invasion (ctEMVI) detection and grading system has been described and validated as a method for predicting disease-free survival (DFS) in colon cancer patients. In this study, we assess the difference in DFS between ctEMVI-positive and -negative colon cancer patients. ctEMVI status as well as pathological T and N status were independent adverse prognostic indicators for colon cancer patients. ctEMVI, in conjunction with the extent of extramural spread and lymph node burden, may become a novel and clinically significant imaging evaluation parameter when deciding whether patients with colon cancer should receive neoadjuvant treatment.
- Citation: Yao X, Yang SX, Song XH, Cui YC, Ye YJ, Wang Y. Prognostic significance of computed tomography-detected extramural vascular invasion in colon cancer. World J Gastroenterol 2016; 22(31): 7157-7165
- URL: https://www.wjgnet.com/1007-9327/full/v22/i31/7157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i31.7157
Colorectal cancer is the third most common cancer and the third leading cause of cancer-related death in the United States[1]. Approximately 39% of colorectal cancer patients have locally advanced disease, and 19% are diagnosed with metastatic disease[2]. The complete surgical resection of tumors with negative margins (R0 resection), lymphadenectomy, and adjuvant chemotherapy are considered to be the standard treatments for patients with locally advanced colon cancer[3]. Large-scale randomized controlled trials have already shown that patients with stage III colorectal cancer benefit from adjuvant chemotherapy after curative surgery[3]. Currently, phases II and III clinical trials are exploring the feasibility of neoadjuvant chemotherapy for colon cancer, which could help with local tumor downstaging and improve the outcomes of patients with high-risk, advanced localized colon cancer[4,5]. As treatment options become more nuanced, preoperative staging and treatment stratification are becoming increasingly important. Advanced tumor status, lymph node metastasis, and deep extramural tumor invasion have been established as high risk factors when staging colorectal cancer. Similarly, the presence of extramural vascular invasion (EMVI) has also proven to be a useful prognostic factor[5-7].
Traditionally, EMVI has been detected histopathologically and defined as tumor tissue within the vessels beyond the muscularis propria. Various studies have shown EMVI to be present in 9% to 61% of colorectal cancers and to be closely associated with local recurrence, distant metastasis, and poor overall survival[8-11]. EMVI can also be visualized on magnetic resonance imaging (MRI) as the infiltration of tumor signal into the extramural vessels (mrEMVI)[12]. A prior study demonstrated that patients who were positive for mrEMVI had significantly higher recurrence rates than patients negative for mrEMVI[13]. Furthermore, having both an mrEMVI-positive status at baseline and after induction treatment was significantly associated with a poor response to neoadjuvant chemoradiation therapy[14]. Use of mrEMVI status combined with the knowledge of adverse features on MRI has the potential to help develop personalized neoadjuvant treatments for patients with rectal cancer.
However, it is difficult to acquire high-resolution MR images allowing for the detailed assessment of local colon cancer characteristics due to imaging technique limitations. Fortunately, contrast-enhanced multiple-row detector computed tomography (MDCT) is currently the standard modality for the evaluation of colon cancer given its short scan time and its ability to allow for convenient three-dimensional reconstructions. Using histopathological results as the gold standard, the accuracy of MDCT-detected EMVI (ctEMVI) is 74% (95%CI: 64-82) with a sensitivity of 78% (95%CI: 65-87), a specificity of 67% (95%CI: 49-81), and a positive predictive value of 81% (95%CI: 68-89)[15]. However, the prognostic significance of ctEMVI status, as detected by computed tomography (CT) in colon cancer, has yet to be explored. In this study, we compared the survival outcomes between ctEMVI-positive and -negative patients to determine whether ctEMVI status can significantly contribute to prognosis-based treatment decisions.
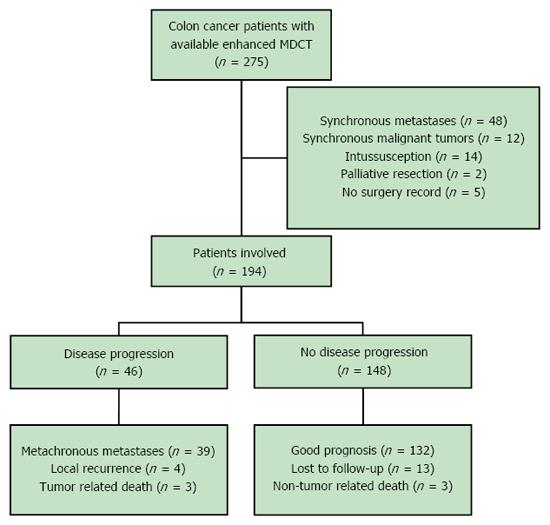
This retrospective study was approved by our local institutional review board, which waived the requirement for obtaining informed consent. Patients were selected from a single hospital’s electronic colon cancer registry. Each patient’s initial clinical and pathological results were dated between January 2009 and December 2013. All included patients had biopsy-proven colon cancer and underwent curative surgery. Additionally, they all underwent MDCT examinations at the following time points: pre-surgery and 3, 6, and 12 mo post-surgery. After the first year, all patients had regular clinical follow-up examinations every 6 mo as well as annual CT examinations. Records were reviewed through December 2015, until the cancer recurred, or until the patient passed away. Patients were excluded for having synchronous metastases, synchronous malignant tumors, intussusception, and/or non-curative surgery.
CT examinations were performed on 64-slice MDCT scanners (Light Speed Volume CT; GE Healthcare, Waukesha, WI, United States). MDCT images were acquired using the following parameters: 120 kV, 240-260 mAs, collimations of 64*0.625, slice thicknesses and increments of 5 mm, and axial reconstructions with 1.25 mm slice thickness and 1 mm slice interval. CT data acquisition of the late atrial and portal venous phases were initiated 10 s and 45 s after the trigger threshold (100 HU on the abdominal aorta) had been reached. Intravenous non-ionic contrast was administrated (100 mL iopromide 370 mg iodine/mL; Bayer Schering Pharma, Berlin, Germany) with a power injector (Missouri XD2001, Ulrich GmbH&Co, Buchbrunnenweg, Ulm, Germany) at a rate of 2.5 mL/s through an antecubital vein. The scanning range began at the inlet of the thorax and ended at the symphysis pubis. Sagittal and coronal reconstructions with 1.25 mm slices were performed on a workstation (Advantage Workstation 4.3; GE Healthcare, Waukesha, WI, United States).
Each biopsy sample or radical surgery specimen was fixed in formalin for 24 h. Hematoxylin and eosin (H&E)-stained slides were then reviewed using a microscope (Olympus BX51, Olympus, Tokyo, Japan) to evaluate the histological type and differentiation of the tumor. Tumor and lymph node statuses of each subject were defined based on the America Joint Committee on Cancer (AJCC) 7th.
The following data were collected: patient demographics (gender and age), preoperative imaging status (EMVI status and tumor location), pathological status [tumor status, lymph node status, and tumor differentiation], and survival outcomes. Tumor and lymph node statuses were categorized according to the criteria of AJCC 7th. Final tumor AJCC stage was determined based on pathology reports and surgery records.
Two board-certified radiologists (5 and 3 years of experience in abdominal radiology, respectively) who were blinded to clinical outcomes and pathology results reviewed all MDCT images and reached a consensus on each one. All radiologists had been trained to evaluate ctEMVI status and had mastered image reconstruction software allowing them to view images on the coronal and sagittal planes as well as on any other planes of interest for a comprehensive analysis. The two radiologists also reviewed all postoperative MDCT images for any disease recurrence.
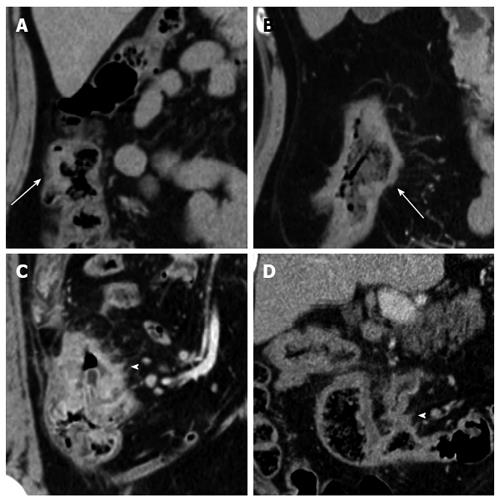
ctEMVI: EMVI was defined as tumor tissue within the vessel wall beyond the muscularis propria. The 4-point ctEMVI grading system used in this study is shown in Table 1 and Figure 1. A score of 0 or 1 corresponded to the absence of ctEMVI, and a score of 2 or 3 corresponded to the presence of ctEMVI.
| CT score | CT status | Morphology features on CT | EMVI status |
| 0 | Definitely no | Absence of tumor extension beyond the colon wall/tumor extension through the colon wall but no adjacent vessels (mesenteric contralateral side) | Negative |
| 1 | No without high confidence | Stranding in proximity of vessels but no tumor density in vessels (mesenteric side) | Negative |
| 2 | Yes without high confidence | Similar tumor density in adjacent vessels; vessel expansion by tumor (mesenteric side) | Positive |
| 3 | Definitely yes | Similar tumor density in adjacent vessels; Irregular vessel contour by tumor (mesenteric side) | Positive |
Tumor location: Tumor locations included the ileocecal junction, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, and sigmoid colon. These locations were divided into two groups: right and left. The right group included the ileocecal junction, ascending colon, hepatic flexure, and the right part of the transverse colon. The left group included the splenic flexure, descending colon, sigmoid colon, and the left part of the transverse colon.
Tumor differentiation: Histological tumor differentiation classifications consisted of adenocarcinoma and mucinous adenocarcinoma. These classifications were further subdivided into well differentiated, moderately differentiated and poorly differentiated.
Pathological tumor status (pT) and lymph node status (pN): Tumor and lymph node status and stage, all in accordance with the criteria AJCC 7th, were determined based on pathological and surgical records.
Median and interquartile ranges were calculated due to an abnormal distribution of data (standardized kurtosis and standardized skewness > 2 and significant Shapiro-Wilks test for normality, P < 0.0001).
Progressive events were defined as radiological or pathological detection of recurrent/metastatic disease or as death caused by primary colon cancer. The rates of progressive events at 1 year and 3 years post-surgery were calculated for the entire cohort, for a subgroup of T4 category colon cancer patients, for a subgroup of patients with stage III colon cancer, and for a subgroup of patients treated with adjuvant chemotherapy. The rates of progressive events between EMVI-positive and -negative groups were analyzed using chi-squared and Fisher’s exact tests.
The outcome measures were 1-year disease-free survival (DFS) and 3-year DFS. DFS was defined as the time from the date of surgery to the date of the local recurrence, and/or distant disease, or tumor-related death. Data on patients who were alive or free from recurrence at the study endpoint, missed follow-up examinations, or died from something other than colon cancer were excluded from DFS analyses. The Kaplan-Meier method was used to estimate survival probabilities in patients with and without EMVI, and the log-rank test was used for statistical comparisons. Cox’s proportional hazards models were developed to test the impact of confounding variables on survival (age, gender, tumor location, pT status, pN status, histological type and tumor differentiation, ctEMVI). Hazard ratios (HR) and 95% confidence interval (CI) were generated.
All statistical tests were two-tailed, and P-values less than 0.05 were considered statistically significant. No correction of P values was applied to adjust for the multiple test issue. However, the results of all statistical tests were thoroughly reported so that an informal adjustment of P values could be performed while reviewing the data. Data were analyzed using SPSS 19.0 (IBM Corporation, Armonk, NY, United States) and MedCalc 11.4 (MedCalc Software bvba, Mariakerke, Belgium).
There were 275 patients who initially met the study inclusion criteria. Forty-eight patients were then excluded for synchronous metastases, 12 for synchronous malignant tumors, 14 for intussusception, two for palliative resection, and five for being lost to follow-up. Ultimately, 194 patients were included in this study (male = 101, female = 93; mean age 67.68 years, range 26-91 years). All patients had undergone curative surgery, and 121 patients had adjuvant chemotherapy after surgery, including 44 stage II patients and 77 stage III patients. All included patients had been routinely monitored with routine follow-up chest/abdomen/pelvis MDCT examinations though December 2015 (Figure 2).
ctEMVI was seen preoperatively in 30.9% (60/194) of the entire cohort. Of the 134 T4 patients, ctEMVI was observed in 54 (40.3%, 54/134) patients. Of the 94 stage III colon cancer patients, ctEMVI was observed in 38 (40.4%, 38/94) patients. Of the 121 patients who underwent adjuvant chemotherapy, ctEMVI was observed in 45 (37.2%, 45/121) patients.
One year after surgery, 16 of 194 (8.2%) patients experienced a progressive event: 14 patients developed metastases, one patient had a local recurrence, and one died as a result of advanced colon cancer. At this time point no statistically significant differences in the rates of progressive events were found between EMVI-positive and -negative patients when evaluating the whole cohort, the T4 subgroup, the stage III subgroup, and the adjuvant chemotherapy subgroup (Table 2).
| ctEMVI status | whole cohort | T4 category patients | Stage III patients | Adjuvant chemotherapy |
| Positive | 11.7% (7/60) | 11.1% (6/54) | 15.8% (6/38) | 11.1% (5/45) |
| Negative | 6.7% (9/134) | 11.3% (9/80) | 10.7% (6/56) | 10.5% (8/76) |
| Odds ratio | 1.8 | 1.0 | 1.6 | 1.1 |
| P value | 0.266 | 0.980 | 0.537 | 1.000 |
At the study endpoint, ctEMVI-positive patients had a significantly higher rate of progressive events than ctEMVI-negative patients within the entire cohort, within the T4 subgroup, within the stage III subgroup, and within the adjuvant chemotherapy subgroup (Table 3). For the whole cohort (n = 194), progressive events occurred in 46 (23.7%, 46/194) patients: 39 patients developed metastases, four patients had local recurrences, and three died of advanced colon cancer.
| ctEMVI status | Whole cohort | T4 category patients | Stage III patients | Adjuvant chemotherapy |
| Positive | 43.3% (26/60) | 42.6% (23/54) | 57.9% (22/38) | 48.9% (22/45) |
| Negative | 14.9% (20/134) | 18.8% (15/80) | 21.4% (12/56) | 19.7% (15/76) |
| Odds ratio | 4.4 | 3.2 | 5 | 3.9 |
| P value | < 0.001 | 0.003 | < 0.001 | 0.001 |
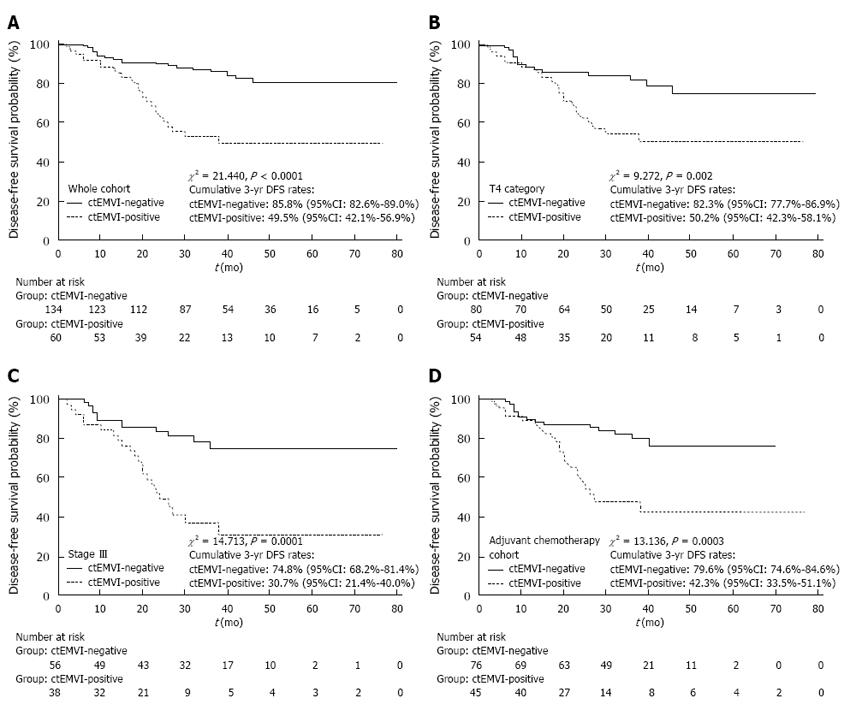
The mean follow-up period was 35.38 mo (95% CI: 32.73-38.02 mo). Based on a Cox proportional hazards regression analysis, ctEMVI was identified as an independent prognostic variable of DFS with an HR of 2.15 (95%CI: 1.12-4.14, P = 0.023) (Table 4). Based on the Kaplan-Meier method, the survival curves were significantly different between ctEMVI-positive and -negative patients in the whole cohort, the T4 subgroup, the stage III subgroup, and the adjuvant chemotherapy subgroup (P < 0.01). The cumulative 3-year DFS rates for ctEMVI-positive and -negative patients are shown in Figure 3A-D.
| Variable | Group | Patient count | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |||
| Gender | Female | 93 | ||||||
| Male | 101 | 1.164 | 0.652-2.078 | 0.606 | 1.288 | 0.714-2.326 | 0.403 | |
| Age (yr) | < 65 | 74 | ||||||
| ≥ 65 | 120 | 1.105 | 0.612-1.997 | 0.742 | 1.618 | 0.838-3.122 | 0.154 | |
| Location | Right | 106 | ||||||
| Left | 88 | 0.820 | 0.458-1.468 | 0.509 | 0.849 | 0.464-1.553 | 0.597 | |
| ctEMVI | Negative | 134 | ||||||
| Positive | 60 | 3.593 | 1.868-6.911 | < 0.0001 | 2.151 | 1.118-4.138 | 0.023 | |
| pT | ≤ T2 | 25 | ||||||
| T3 | 35 | |||||||
| T4a | 105 | |||||||
| T4b | 29 | N/A | N/A | < 0.0001 | 1.876 | 1.161-3.029 | 0.011 | |
| pN | N0 | 100 | ||||||
| N1 | 58 | |||||||
| N2 | 36 | N/A | N/A | < 0.0001 | 2.031 | 1.391-2.966 | 0.000 | |
| D | Well/moderate | 136 | ||||||
| Poor | 58 | 1.453 | 0.767-2.751 | 0.217 | 1.006 | 0.527-1.920 | 0.985 | |
The main finding of this study is that the cumulative 3-year DFS rates significantly differ between patients with and without EMVI as seen in the subgroups of patients with T4 colon cancer, with stage III colon cancer, and with adjuvant chemotherapy after curative surgery. Moreover, ctEMVI is an independent prognostic variable that predicts cumulative 3-year DFS with an HR of 2.15 (95%CI: 1.12-4.14, P = 0.023).
Since 1980, EMVI in colorectal cancer had been reported as an adverse prognostic indicator in previous histopathological studies[10,16]. Courtney et al[9] found that EMVI could be used to predict progressive events in a prospective cohort of 378 patients with colorectal cancer based on histopathological analyses after curative surgery: EMVI was present in 28.3% (107/378) of cases and significantly reduced 5-year overall survival compared to patients without EMVI. However, there is little literature available about the relationship between EMVI status, as preoperatively assessed by MDCT, and the prognosis of colon cancer.
In the present study, ctEMVI was demonstrated as an independent factor predicting cumulative 3-year DFS of the whole cohort of patients with colon cancer. In general, tumor infiltration and lymph node metastasis have proven to be closely associated with progressive events in patients with colon cancer. ctEMVI scores achieved a level of independent prognostic significance in multivariable analyses comparable to the level achieved by established prognostic indicators, such as pathologically defined tumor depth infiltration and lymph node burden. However, our findings are limited by the fact that in our study EMVI, as detected by MDCT, could not be confirmed by pathological analyses due to the retrospective nature of this study. Furthermore, when analyzing a subgroup only including T4 category colon cancers, a significantly lower cumulative 3-year DFS rate was seen in patients with ctEMVI-positive colon cancer compared to ctEMVI-negative colon cancer. When analyzing a subgroup of patients with stage III colon cancer and metastatic lymph nodes, ctEMVI-positive colon cancer patients had a significantly higher recurrence rate compared to ctEMVI-negative colon cancer patients. Moreover, patients who underwent adjuvant chemotherapy had significantly different 3-year DFS outcomes based on their EMVI status.
This study is clinically relevant because it shows that patients can be risk-stratified before neoadjuvant chemotherapy based on their ctEMVI status and then can be advised accordingly. The findings of the randomized controlled trial FOxTROT showed that neoadjuvant chemotherapy for locally advanced operable primary colon cancer was feasible with acceptable toxicity and perioperative morbidity. Meanwhile, this randomized trial emphasized that only patients with high-risk factors, including extramural invasion depth ≥ 5 mm or EMVI, be selected for neoadjuvant chemotherapy so that overtreatment of patients with low risk factors can be avoided[4]. When deciding whether to administer neoadjuvant therapy in patients with locally advanced colon cancer, it has been found that an multidisciplinary team should consider the following adverse risk factors: extramural spread, lymph node burden, and especially extramural venous invasion[17,18].
It should be noted in the present study that no significant difference in DFS between ctEMVI-positive and -negative patients was seen 1 year after the study start point. Similarly, no differences in 1-year disease recurrence rates were found between ctEMVI-positive and -negative patients in the entire cohort, the stage III subgroup, and the subgroup of patients treated with adjuvant chemotherapy. This finding is in contrast to a study by Bugg et al[17] who demonstrated a significant difference in metastatic rates between rectal cancer patients with (24.5%, 13/53) and without mrEMVI (6.7%, 10/149) at a 1-year follow-up examination with an odds ratio of 3.7. In addition, the 1-year disease relapse rate was also obtained in patients with stage III gastric cancer in another study done by our team[19]. The results of the present study suggest that EMVI-positive colon cancer may be less aggressive than rectal cancer or gastric cancer with similar features, but, ultimately, EMVI is an adverse imaging feature.
This study was limited by its retrospective nature. Because EMVI status was not recorded routinely by our institution’s pathology department, the accuracy of the ctEMVI values could not be evaluated against the gold standard of histopathology. The fact that the pathology department lacks a policy regarding EMVI speaks to the absence of and the need for formal guidelines regarding the pathologic assessment of vascular invasion and the application of specific reporting criteria in our hospital. Similarly, preoperative radiological tumor stage, lymph node burden, and even extramural vascular invasion have not been well established on MDCT imaging even though MDCT imaging is currently the standard for evaluating colon cancer because of its short scan time and its ability to be conveniently reconstructed into a three dimensional image[20]. In order to standardize radiological and pathological criteria for evaluating colon cancer, we advise that workshops be established on staging colon cancer for both gastrointestinal radiologists and pathologists. Furthermore, future prospective studies are needed to evaluate how accurate pathologists and radiologists are at detecting EMVI to inform the content of said workshops. Establishing diagnostic criteria for EMVI will contribute to more appropriate treatments for colon cancer patients.
In conclusion, EMVI, as detected on contrast-enhanced multiple-row detector CT, is an independent prognostic factor for colon cancer. ctEMVI, in conjunction with the extent of extramural spread and lymph node burden, may also become a novel and clinically significant imaging evaluation parameter when deciding whether patients with colon cancer should receive neoadjuvant treatment or not.
We would like to thank Chun-Fang Zhang for her assistance with the statistical analyses for this study.
Colorectal cancer is the third most common cancer and the third leading cause of cancer-related death in the United States. The complete surgical resection of tumors with negative margins (R0 resection), lymphadenectomy, and adjuvant chemotherapy are considered to be the standard treatments for patients with locally advanced colon cancer. As treatment options become more nuanced, preoperative staging and treatment stratification are becoming increasingly important. Extramural vascular invasion (EMVI) in colorectal cancer had been reported as an adverse prognostic indicator in histopathology. The purpose of this study was to assess the difference in disease-free survival (DFS) of patients with colon cancer between CT-detected EMVI (ctEMVI) -positive and -negative groups.
ctEMVI is important for risk stratification of colon cancer patients. Few prior studies have concentrated on the relationship between ctEMVI and the prognosis of colon cancer. The results of this study help demonstrate the importance of ctEMVI for evaluating disease progression and for developing an individualized treatment plan for colon cancer.
In this study, ctEMVI was demonstrated to be an independent factor in predicting disease progression with an HR of 2.15 (95%CI: 1.12-4.14, P = 0.023). The cumulative 3-year DFS rates were 49.5% (95%CI: 42.1%-56.9%) and 85.8% (95%CI: 82.6-89.0) for ctEMVI-positive and -negative patients in the whole cohort, respectively (χ2 = 21.440, P < 0.0001). The results are in agreement with previous studies on MRI-detected EMVI (mrEMVI) in rectal cancer. However, there is little literature available about the relationship between ctEMVI status and the prognosis of colon cancer.
This study suggests that ctEMVI is useful for evaluating disease progression in colon cancer patients. Positive ctEMVI may become a novel and clinically significant imaging evaluation parameter when deciding whether colon cancer patients should receive neoadjuvant treatment.
The manuscript presented by Wang et al is of practical interest. The authors evaluated the efficacy of ctEMVI for evaluating disease progression by comparing the progressive event rates and DFS between ctEMVI-positive and -negative groups and by conducting univariate and multivariate survival analyses for DFS for colon cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bester L, Bonin S S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2070] [Article Influence: 188.2] [Reference Citation Analysis (0)] |
| 2. | Kurniali PC, Hrinczenko B, Al-Janadi A. Management of locally advanced and metastatic colon cancer in elderly patients. World J Gastroenterol. 2014;20:1910-1922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Benson AB, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton MJ. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:519-528. [PubMed] |
| 4. | Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Dighe S, Swift I, Magill L, Handley K, Gray R, Quirke P, Morton D, Seymour M, Warren B, Brown G. Accuracy of radiological staging in identifying high-risk colon cancer patients suitable for neoadjuvant chemotherapy: a multicentre experience. Colorectal Dis. 2012;14:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Chand M, Swift RI, Tekkis PP, Chau I, Brown G. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2014;110:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Bhangu A, Fitzgerald JE, Slesser A, Northover JM, Faiz O, Tekkis P. Prognostic significance of extramural vascular invasion in T4 rectal cancer. Colorectal Dis. 2013;15:e665-e671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Chand M, Palmer T, Blomqvist L, Nagtegaal I, West N, Brown G. Evidence for radiological and histopathological prognostic importance of detecting extramural venous invasion in rectal cancer: recommendations for radiology and histopathology reporting. Colorectal Dis. 2015;17:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Courtney ED, West NJ, Kaur C, Ho J, Kalber B, Hagger R, Finlayson C, Leicester RJ. Extramural vascular invasion is an adverse prognostic indicator of survival in patients with colorectal cancer. Colorectal Dis. 2009;11:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, Vieth M, Hoefler G, Langner C. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer. 2012;118:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 11. | Dresen RC, Peters EE, Rutten HJ, Nieuwenhuijzen GA, Demeyere TB, van den Brule AJ, Kessels AG, Beets-Tan RG, van Krieken JH, Nagtegaal ID. Local recurrence in rectal cancer can be predicted by histopathological factors. Eur J Surg Oncol. 2009;35:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191:1517-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 14. | Chand M, Evans J, Swift RI, Tekkis PP, West NP, Stamp G, Heald RJ, Brown G. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg. 2015;261:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | Dighe S, Blake H, Koh MD, Swift I, Arnaout A, Temple L, Barbachano Y, Brown G. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. Br J Surg. 2010;97:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5:141-163. [PubMed] |
| 17. | Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. 2014;69:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Dewdney A, Cunningham D, Chau I. Selecting patients with locally advanced rectal cancer for neoadjuvant treatment strategies. Oncologist. 2013;18:833-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Cheng J, Wu J, Ye Y, Zhang C, Zhang Y, Wang Y. The prognostic significance of extramural venous invasion detected by multiple-row detector computed tomography in stage III gastric cancer. Abdom Radiol. 2016;41:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Smith NJ, Bees N, Barbachano Y, Norman AR, Swift RI, Brown G. Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: implications for clinical trials. Br J Cancer. 2007;96:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |