Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6841
Peer-review started: April 2, 2016
First decision: May 12, 2016
Revised: June 9, 2016
Accepted: July 6, 2016
Article in press: July 6, 2016
Published online: August 14, 2016
Processing time: 128 Days and 19.1 Hours
The hepatic stellate cells in the liver are stimulated sustainably by chronic injury of the hepatocytes, activating myofibroblasts, which produce abundant collagen. Myofibroblasts are the major source of extracellular proteins during fibrogenesis, and may directly, or secreted products, contribute to carcinogenesis and tumor progression. Cancer-associated fibroblasts (CAFs) are one of the components of the tumor microenvironment that promote the proliferation and invasion of cancer cells by secreting various growth factors and cytokines. CAFs crosstalk with cancer cells stimulates tumor progression by creating a favorable microenvironment for progression, invasion, and metastasis through the epithelial-mesenchymal transition. Basic studies on CAFs have advanced, and the role of CAFs in tumors has been elucidated. In particular, for hepatocellular carcinoma, carcinogenesis from cirrhosis is a known fact, and participation of CAFs in carcinogenesis is supported. In this review, we discuss the current literature on the role of CAFs and CAF-related signaling in carcinogenesis, crosstalk with cancer cells, immunosuppressive effects, angiogenesis, therapeutic targets, and resistance to chemotherapy. The role of CAFs is important in cancer initiation and progression. CAFtargeted therapy may be effective for suppression not only of fibrosis but also cancer progression.
Core tip: Cancer-associated fibroblasts (CAFs) are one of the most crucial components of the tumor microenvironment that promote the carcinogenesis, proliferation and invasion of cancer cells by secreting various growth factors and cytokines. In hepatocellular carcinoma (HCC), cirrhosis caused by chronic inflammation was considered the main reason for carcinogenesis, and field cancerization was explained by the epigenetic changes in fibroblasts in tissues surrounding the tumor. In this review, we discuss the findings from current literature on the role of CAFs in HCC. CAF-targeted therapy may be effective for suppression not only fibrosis but also cancer progression.
- Citation: Kubo N, Araki K, Kuwano H, Shirabe K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J Gastroenterol 2016; 22(30): 6841-6850
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6841.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6841
Hepatocellular carcinoma (HCC) is the second most common cause of death from cancer worldwide, and accounted for nearly 746000 deaths in 2012[1]. Malignant tumors comprise cancer cells and stromal cells. For a long time, the malignant potential of the tumor was thought to be entirely due to the cancer cells because the stromal cells were not undergoing differentiation or activate proliferation[2]. Stromal cells were considered to simply surround the cancer cells and have a non-malignant function. Recent studies have clarified the origins, features, and roles of the stromal cells.
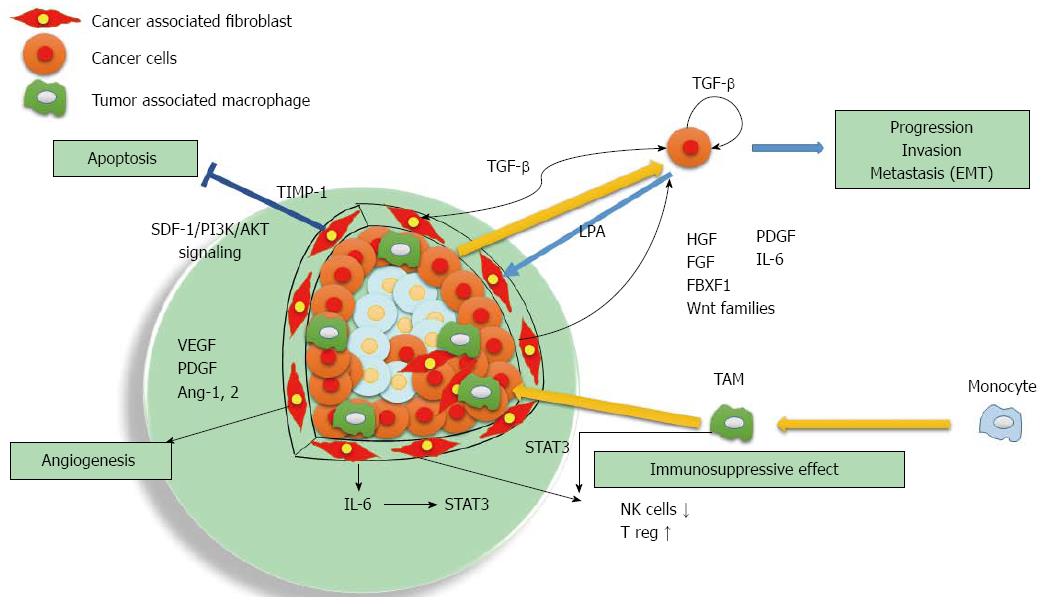
Fibroblasts in cancer tissues are similar in morphology to the myofibroblasts that are activated during the wound healing process[3]. Recent studies have shown the importance of cross talk between cancer cells and the fibroblasts called cancer-associated fibroblasts (CAFs)[4]. CAFs are active in a wound healing process similar to normal myofibroblasts[5], and promote tumor proliferation, invasion, and metastasis via secretion of various growth factors, cytokines and degradation of extracellular matrix proteins[6-8] (Figure 1).
CAFs are large spindle-shaped mesenchymal cells with positive immunostaining for vimentin, alpha smooth muscle actin, and developed fibronexus[9,10]. The types of surface marker proteins on CAFs and non-tumoral fibroblasts (NTFs), which are primary cells from cirrhotic tissue, can be detected by flow cytometry and immunofluorescence[4]. No significant difference between CAFs and NTFs in surface markers is evident, but mRNA expression of αSMA is higher in CAFs compared to NTFs[4]. It has been reported that DNA-methylation-based epigenetic changes have already occurred in activated hepatic stellate cells (HSC)[11,12]. Therefore, NTF, which are activated HSCs from cirrhotic tissue, may have already undergone a genetic change that affects surface markers. Collagen 11A1 expression is a remarkable biomarker of human carcinoma-associated stromal cells[13]. It was reported that even without exposure to cancer cells, the tumor promoting characteristics of CAFs can be stably maintained[14]. There remains a persistent risk for HCC in patients with advanced fibrosis who have achieved a sustained virologic response (SVR)[15]. These observations indicate that genetic or epigenetic changes may have already existed in the CAFs independent of the original tumor[14]. Furthermore, activated HSCs can be stimulated by cancer cells, which then become CAFs. Details of differentiation into quiescent HSCs, HSCs, and CAFs is shown in the Table 1. Quiescent HSCs were characterized by the stored vitamin A with fat droplets[16] and are derived from the mesoderm[17]. HSCs have a function in wound healing and fibroblast production. Characterization of CAFs revealed that they were affected by cancer cells through “crosstalk”.
| Quiescent hepatic stellate cells | Hepatic stellate cells | Cancer associated | |
| (fibroblast) | (myofibroblast) | fibroblasts | |
| Morphology | Spindle shape with numerous intracellular droplets[16] | Spindle shape | Spindle shape |
| Origin | Mesoderm[17] | Quiescent hepatic stellate cells | Activated hepatic stellate cells |
| Location | Space of Disse, sinusoidal spaces | Periportal lesion | Tumor stroma |
| Biological markers | Desmin[17] | αSMA, p75NTR[17] | αSMA, COL11A1[13] |
| Function | Store the vitamin A and fat | Wound healing fibrosis | Tumor progression |
| Cytokines | - | Production of the collagens, PDGF, TGF-β, TNF-α, IL-6, IL-1β[25,26] | production of the collagens, TGF-β, HGF, FGF, VEGF, IL-6[42-58], etc |
It is well established in other systems that complex intercellular signaling networks exist between tumors and CAFs, contributing to cancer initiation, growth, and progression[18-22]. Tumor secretion of cytokines, such as transforming growth factor-β (TGF-β), stimulate myofibroblast activation leading to profound changes in extracellular matrix composition and organization. The role of mesenchymal stroma alterations in cancer initiation was proposed in the context of colon[23] and prostate[24] cancers. Chronic liver injuries caused by viral hepatitis, autoimmune hepatitis, alcoholic hepatitis, and non-alcoholic steatohepatitis activate and transform quiescent fibroblasts into activated myofibroblasts through the actions of increased growth factors and continued expression of inflammatory cytokines such as PDGF, TGF-β, TNF-α, IL-6, and IL-1β[25,26]. Fibroblasts might be directly activated by hepatitis C infection, which leads to production of reactive oxygen species and TGF-β[27]. The activated fibroblasts produce massive amounts of extracellular matrix proteins, including type I collagen, which leads to liver fibrosis[28]. Persistent inflammation in chronic hepatitis plays a major role in the development of HCC[29-31]. There remains a persistent risk for HCC in patients with advanced fibrosis who have achieved SVR[15]. Around 90% of HCC cases are associated with fibrotic or cirrhotic livers[32,33].
Dotto[34] suggested that while changes in tumor stroma are frequently viewed as secondary to changes in the epithelium, recent evidence indicates that they can play a primary role in both cancer progression and initiation. These changes include epigenetic events such as loss of p53[35-37]. These processes may explain the phenomenon of field cancerization, namely the occurrence of multifocal and recurrent epithelial tumors that are preceded by and associated with widespread changes of surrounding tissue or organ fields[34]. It thought that epigenetic changes affect CAFs in HSCs in cases of liver cirrhosis that include abundant fibrosis. However, gene analysis is not performed often enough in human HSCs of the liver cirrhosis or CAFs[38].
Recent studies have shown the importance of crosstalk between cancer cells and their stromal microenvironment, including HCC[4,39,40]. CAFs are the most important cell type in the stroma and play a critical role in modulating neighboring cancer cells[41]. CAFs stimulate malignant cell proliferation by providing different types of growth factors and cytokines in a context-dependent manner[20] such as SDF-1[42-45], HGF[46-48], members of the epidermal growth factor family[49], fibroblast growth factor (FGF)[50,51], Wnt families[52], forkhead box F1[53], IL-6[54-56], TGF-β[57,58], and EGF. When HCC cells are co-cultured with CAFs, CAFs induced by TIMP-1 repress HCC apoptosis with an increased Bcl-2/BAX ratio through SDF-1/CXCR4/PI3K/AKT signaling[44]. Moreover, CAFs upregulated gene expressions of TGF-β and FAP, whereas NTFs did not induce the expression of either gene[4]. HGF is expressed by CAFs, HSCs, and myofibroblasts[48,59,60], and it is a highly potent hepatocyte growth factor regulating cell proliferation, migration, survival, and angioneogenesis[61-64]. IL-6 stimulated progranulin expression contributes to the malignancy of HCC cells by activating mTOR signaling[56]. After the IL-6/STAT3 pathway is activated, malignant cells proliferate much faster and their anti-apoptosis ability increases significantly[65]. TGF-β complex is secreted by most cell types, including human HSCs and hepatocytes[66,67]. TGF-β signaling promotes HCC progression by two mechanisms: first, via an intrinsic activity as an autocrine or paracrine growth factor, and second, via an extrinsic activity by inducing microenvironment changes, including CAFs activation, T regulatory cell increases, and inflammatory mediators[68]. A recent study using transgenic mice suggested that PDGF-C overexpressing hepatocytes causes activation of HSC, which in turn produces HGF and cytokines, resulting in the development of HCC[69]. Crosstalk between TGF-β and PDGF signaling supports epithelial mesenchymal transition (EMT), which is crucial for tumor growth and the acquisition of an invasive phenotype[70]. MRC-5 fibroblast-conditioned medium influences multiple pathways regulating invasion in HCC[71].
Lysophosphatidic acid (LPA) is a lipid mediator that is involved in multiple cellular events associated with tumor initiation and progression, invasion, and metastasis[72,73]. LPA is secreted by HCC cells and promotes transdifferentiation of myofibroblasts by the paracrine mechanism and has been clearly shown to be a therapeutic target for tumor-CAF interactions in HCC[41,74]. MMP-9 is downstream of LPA and has been postulated to have a critical role in HCC cell invasion and metastasis[73] by secreting various matrix-degrading proteases as well as their activators such as uPA[75].
These functions of CAFs in supporting HCC growth were confirmed by in vitro experiments involving coculture of HCC cell lines with CAFs[4]. Remarkably, the activation of CAFs was maintained after their isolation from cells of various cancer types such as squamous skin carcinoma, lung carcinoma, breast carcinoma, and scirrhous gastric cancer[76-78]. Exposure to leukemia inhibitory factor initiates an epigenetic switch causing the constitutive activation of JAK1/STAT3 signaling, which results in sustained activation of CAFs[79]. DNA methylation plays critical roles in the control of sustained and constitutive activation of signaling pathways[80]. CAF activation is accompanied by stromal cell senescence[81,82]. Concomitant loss of CSL (also known as RBP-Jk) and p53 overcomes fibroblast senescence, enhances expression of CAF effectors, and promotes stromal and cancer cell expansion[81] through β-galactosidase[83], IL-6, and IL-8[82] respectively. Xenografts in nude mice also demonstrated in vivo tumor growth enhancement by CAFs[48].
There is an impaired anti-tumor response within the HCC microenvironment due to various immune suppressive elements[84], including regulatory T cells (Tregs)[85], tumor-associated macrophages (TAMs)[86], and tumor-associated neutrophils (TANs)[87,88]. Among the immune cell types present within the HCC, TAMs play a leading role in the setting of the crosstalk between tumor and stromal cells[89]. TAMs are mainly polarized towards an M2 phenotype, which is a major component of leukocyte infiltration of tumors and plays a pivotal role in tumor progression of HCC[90]. Increased TAMs are correlated with angiogenesis, metastasis, and poor prognosis[91-94]. STAT3 activation is correlated with aggressive behavior of HCC and may be mediated via TAMs[95].
CAFs educate NK cells to acquire a deactivated phenotype and create an unresponsive condition in tumors[96]. This suppression is eliminated by indoleamine 2,3dioxygenase (IDO) and/or PGE2 inhibitors[96]. CAFs recruit regulatory dendritic cells and educate them to acquire a tolerogenic phenotype through IL-6 mediated STAT3 activation[97] and upregulate the production of Tregs by secreting TGFβ in tumor microenvironments[98]. The mechanisms underlying CAFs’ immunomodulatory effects in HCC may be mediated via upregulation of human B7 homolog 1 (B7-H1) in CAFs[99]. B7-H1/programmed death 1 (PD-1) signaling promotes Treg cell induction and immunosuppressive function through the down regulation of mTOR and AKT phosphorylation[100]. Using these immunosuppression effects, CAFs that receive cytokine signals from cancer cells produce an environment that is convenient for cancer cells.
A hypoxic condition activates Akt, which increases the expression of vascular endothelial growth factor (VEGF), the most important angiogenic factor. The rapid growth of the HCC requires new vessels. CAFs secrete angiogenic factors, including VEGF, PDGF, MMPs, FDF, TGF-β1, EGF, angiopoietin-1, and angiopoietin-2, which have a critical role in HCC initiation, progression, and metastasis, and creates new vessels[101-105]. VEGF receptor, PDGF receptor, and Tie-2 upregulation also occur during CAFs activation, resulting in increased mitogenesis in response to VEGF[19,106-108]. VEGF secretion by HSCs can be hormonally induced by leptin, or by physical stress such as hypoxia, and is upregulated in HCC[104,106,109]. Conditioned medium from HCC cells can activate CAFs and stimulate VEGF production. Oxidative stress enhances the malignant potential of HCC through the stimulation of angiogenesis by activation of the Akt-VEGF pathway[110]. Angiogenesis is also facilitated by TAM-derived proteases because extracellular proteolysis is necessary for new vessel formation. The most prominent proteinases that promote tumor-directed angiogenesis include matrix metalloproteinase, plasmin, and urokinase-type plasminogen activator and its receptor[111,112].
Anti-cancer therapy targeting CAFs or inhibitors of the cytokines secreted by CAFs has been actively investigated recently. Inhibitors of TGF-β signaling have been shown to block HCC growth and progression by modulating EMT in different experimental models, leading to the clinical investigation of a TGF-β inhibitor monohydrate in HCC[68]. Several receptor tyrosine kinase inhibitors target VEGF and PDGF. Linifanib is a potent inhibitor of VEGF, PDGF, PDGFR-β, KDR, and colony stimulating factor-1-receptor (CSF). Sunitinib inhibits receptors for PDGF and VEGF, as well as other receptor tyrosine kinases such as CSF. Some groups have explored active targeting of CAFs to deliver therapeutic compounds. This involves coupling the selected compound to a carrier possessing a specific receptor binding ligand or an antibody. Carriers employed have included an antibody to the synaptophysin receptor on CAFs, and a liposome specific to the vitamin A receptor on CAFs[68,113,114].
These therapeutic targets are several cytokines secreted by CAFs or signals from CAFs that stimulate the HCC. Liver fibrosis is treated with anti-fibrotic drugs that inhibit the activation of quiescent HSCs and promote cell death in activated HSC. If CAFs are an activated state of HSC, it is possible that these drugs were effective for CAFs. A recent report suggested some anti-fibrotic drugs, such as PRI-724[115], conophylline[116], armepavine[117], follistatin[118], salvianolic acids[119], ursolic acid[120], gliotoxin[121], curcumin[122], sulfasalazine[123], benzodiazepine[124] and tanshinone I[125], which suppress activated HSCs and/or induce apoptosis. It is thought that these drugs not only control the fibrosis, but also suppress the HCC by controlling the function of CAFs. Restraining fibrosis may lead to controlling further carcinogenesis according to the theories about field cancerization.
Fibrolamellar HCC was surrounded by laminated fibrous stroma[126]. It was reported the overexpression of fibroblast growth factor receptor 1 in fibrolamellar HCC[127]. CAFs stimulate tumor cells by FGF[50,51] and produced fibrosis. Treatment targeting CAFs is might be effective in a fibrolamellar HCC.
CAFs stimulate malignant cell proliferation by providing different types of growth factors and cytokines in a context-dependent manner[20] such as SDF-1[42-45], HGF[46-48], members of the epidermal growth factor family[49], fibroblast growth factor (FGF)[50,51].
After undergoing EMT, malignant cells become more resistant to chemotherapy, and those expressing surface molecules of stem cells increase, suggesting a close link between CAF-induced EMT, tumor stem cells, and chemoresistance of tumor cells[128,129]. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts[130]. Tumors with stromal phenotypes are more chemoresistant and share more characteristics with tumor stem cells[131]. CAFs are primarily resistant to chemotherapy due to a small proportion of proliferating cells in contrast to malignant cells[132]. CAFs induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells[133]. CAFs attenuate the sensitivity to cisplatin in ovarian cancer cells by promoting STAT3 signaling[134]. These findings about a connection between CAFs and chemoresistance are from recent studies, and the mechanisms of resistance are still unclear.
CAFs are one of the most crucial components of the tumor microenvironment that promote the growth and invasion of cancer cells by various mechanisms. Chronic inflammation was previously considered the main reason for carcinogenesis leading to HCC. However, there remains a persistent risk for HCC in patients with advanced fibrosis who have achieved SVR[15]. These results suggest that procancer stromal alterations were made by the CAFs and CAFs related cells. In conclusion, CAFs are important for cancer cell initiation and progression, and therapy targeting CAFs may be effective for treating fibrosis and preventing HCC progression.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kelleher FC, Cui JF S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Thompson AI, Conroy KP, Henderson NC. Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol. 2015;15:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers (Basel). 2015;7:2443-2458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 592] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 3. | De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 531] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 4. | Sukowati CH, Anfuso B, Crocé LS, Tiribelli C. The role of multipotent cancer associated fibroblasts in hepatocarcinogenesis. BMC Cancer. 2015;15:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2964] [Cited by in RCA: 3149] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 6. | Terada T, Makimoto K, Terayama N, Suzuki Y, Nakanuma Y. Alpha-smooth muscle actin-positive stromal cells in cholangiocarcinomas, hepatocellular carcinomas and metastatic liver carcinomas. J Hepatol. 1996;24:706-712. [PubMed] |
| 7. | Yamamura Y, Asai N, Enomoto A, Kato T, Mii S, Kondo Y, Ushida K, Niimi K, Tsunoda N, Nagino M. Akt-Girdin signaling in cancer-associated fibroblasts contributes to tumor progression. Cancer Res. 2015;75:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Song J, Ge Z, Yang X, Luo Q, Wang C, You H, Ge T, Deng Y, Lin H, Cui Y. Hepatic stellate cells activated by acidic tumor microenvironment promote the metastasis of hepatocellular carcinoma via osteopontin. Cancer Lett. 2015;356:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med. 2008;12:22-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 723] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 11. | El Taghdouini A, Sørensen AL, Reiner AH, Coll M, Verhulst S, Mannaerts I, Øie CI, Smedsrød B, Najimi M, Sokal E. Genome-wide analysis of DNA methylation and gene expression patterns in purified, uncultured human liver cells and activated hepatic stellate cells. Oncotarget. 2015;6:26729-26745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Martin M, Ancey PB, Cros MP, Durand G, Le Calvez-Kelm F, Hernandez-Vargas H, Herceg Z. Dynamic imbalance between cancer cell subpopulations induced by transforming growth factor beta (TGF-β) is associated with a DNA methylome switch. BMC Genomics. 2014;15:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Vázquez-Villa F, García-Ocaña M, Galván JA, García-Martínez J, García-Pravia C, Menéndez-Rodríguez P, González-del Rey C, Barneo-Serra L, de Los Toyos JR. COL11A1/(pro)collagen 11A1 expression is a remarkable biomarker of human invasive carcinoma-associated stromal cells and carcinoma progression. Tumour Biol. 2015;36:2213-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Littlepage LE, Egeblad M, Werb Z. Coevolution of cancer and stromal cellular responses. Cancer Cell. 2005;7:499-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Hendriks HF, Verhoofstad WA, Brouwer A, de Leeuw AM, Knook DL. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985;160:138-149. [PubMed] |
| 17. | Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2009;49:998-1011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Henderson NC, Iredale JP. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci (Lond). 2007;112:265-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2193] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 20. | Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1649] [Cited by in RCA: 1726] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 21. | Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002-5011. [PubMed] |
| 22. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4107] [Article Influence: 205.4] [Reference Citation Analysis (3)] |
| 24. | Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 279] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Török NJ. Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol. 2008;43:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol. 2009;36:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Schuppan D, Krebs A, Bauer M, Hahn EG. Hepatitis C and liver fibrosis. Cell Death Differ. 2003;10 Suppl 1:S59-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA. New aspects of hepatic fibrosis. J Hepatol. 2000;32:32-38. [PubMed] |
| 29. | Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173-S181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 371] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 30. | Li C, Deng M, Hu J, Li X, Chen L, Ju Y, Hao J, Meng S. Chronic inflammation contributes to the development of hepatocellular carcinoma by decreasing miR-122 levels. Oncotarget. 2016;7:17021-17034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Wallace MC, Friedman SL. Hepatic fibrosis and the microenvironment: fertile soil for hepatocellular carcinoma development. Gene Expr. 2014;16:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6560] [Article Influence: 468.6] [Reference Citation Analysis (1)] |
| 33. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3587] [Article Influence: 275.9] [Reference Citation Analysis (4)] |
| 34. | Dotto GP. Multifocal epithelial tumors and field cancerization: stroma as a primary determinant. J Clin Invest. 2014;124:1446-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Hu M, Yao J, Cai L, Bachman KE, van den Brûle F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 36. | Jiang L, Gonda TA, Gamble MV, Salas M, Seshan V, Tu S, Twaddell WS, Hegyi P, Lazar G, Steele I. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68:9900-9908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 38. | Lin ZY, Chuang WL. Hepatocellular carcinoma cells cause different responses in expressions of cancer-promoting genes in different cancer-associated fibroblasts. Kaohsiung J Med Sci. 2013;29:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Kim GJ, Rhee H, Yoo JE, Ko JE, Lee JS, Kim H, Choi JS, Park YN. Increased expression of CCN2, epithelial membrane antigen, and fibroblast activation protein in hepatocellular carcinoma with fibrous stroma showing aggressive behavior. PLoS One. 2014;9:e105094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Eiró N, Vizoso FJ. Importance of tumor/stroma interactions in prognosis of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2014;3:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 41. | Mazzocca A, Dituri F, Lupo L, Quaranta M, Antonaci S, Giannelli G. Tumor-secreted lysophostatidic acid accelerates hepatocellular carcinoma progression by promoting differentiation of peritumoral fibroblasts in myofibroblasts. Hepatology. 2011;54:920-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2625] [Cited by in RCA: 2884] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 43. | Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Song T, Dou C, Jia Y, Tu K, Zheng X. TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget. 2015;6:12061-12079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Teng F, Tian WY, Wang YM, Zhang YF, Guo F, Zhao J, Gao C, Xue FX. Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. J Hematol Oncol. 2016;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 46. | Amann T, Bataille F, Spruss T, Mühlbauer M, Gäbele E, Schölmerich J, Kiefer P, Bosserhoff AK, Hellerbrand C. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009;100:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 47. | Li Q, Wang W, Yamada T, Matsumoto K, Sakai K, Bando Y, Uehara H, Nishioka Y, Sone S, Iwakiri S. Pleural mesothelioma instigates tumor-associated fibroblasts to promote progression via a malignant cytokine network. Am J Pathol. 2011;179:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Jia CC, Wang TT, Liu W, Fu BS, Hua X, Wang GY, Li TJ, Li X, Wu XY, Tai Y. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS One. 2013;8:e63243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q, Yuan HF, Nan X, Chen HX, Zhou JN, Lin YL. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2012;132:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 620] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 51. | Giulianelli S, Cerliani JP, Lamb CA, Fabris VT, Bottino MC, Gorostiaga MA, Novaro V, Góngora A, Baldi A, Molinolo A. Carcinoma-associated fibroblasts activate progesterone receptors and induce hormone independent mammary tumor growth: A role for the FGF-2/FGFR-2 axis. Int J Cancer. 2008;123:2518-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Fu L, Zhang C, Zhang LY, Dong SS, Lu LH, Chen J, Dai Y, Li Y, Kong KL, Kwong DL. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/β-catenin signalling pathway. Gut. 2011;60:1635-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 53. | Saito RA, Micke P, Paulsson J, Augsten M, Peña C, Jönsson P, Botling J, Edlund K, Johansson L, Carlsson P. Forkhead box F1 regulates tumor-promoting properties of cancer-associated fibroblasts in lung cancer. Cancer Res. 2010;70:2644-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Paland N, Kamer I, Kogan-Sakin I, Madar S, Goldfinger N, Rotter V. Differential influence of normal and cancer-associated fibroblasts on the growth of human epithelial cells in an in vitro cocultivation model of prostate cancer. Mol Cancer Res. 2009;7:1212-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Lin ZY, Chuang YH, Chuang WL. Cancer-associated fibroblasts up-regulate CCL2, CCL26, IL6 and LOXL2 genes related to promotion of cancer progression in hepatocellular carcinoma cells. Biomed Pharmacother. 2012;66:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Liu F, Zhang W, Yang F, Feng T, Zhou M, Yu Y, Yu X, Zhao W, Yi F, Tang W. Interleukin-6-stimulated progranulin expression contributes to the malignancy of hepatocellular carcinoma cells by activating mTOR signaling. Sci Rep. 2016;6:21260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3347] [Cited by in RCA: 3523] [Article Influence: 185.4] [Reference Citation Analysis (1)] |
| 58. | Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed). 2010;15:166-179. [PubMed] |
| 59. | Guirouilh J, Castroviejo M, Balabaud C, Desmouliere A, Rosenbaum J. Hepatocarcinoma cells stimulate hepatocyte growth factor secretion in human liver myofibroblasts. Int J Oncol. 2000;17:777-781. [PubMed] |
| 60. | Guirouilh J, Le Bail B, Boussarie L, Balabaud C, Bioulac-Sage P, Desmoulière A, Schuppan D, Rosenbaum J. Expression of hepatocyte growth factor in human hepatocellular carcinoma. J Hepatol. 2001;34:78-83. [PubMed] |
| 61. | Efimova EA, Glanemann M, Liu L, Schumacher G, Settmacher U, Jonas S, Langrehr JM, Neuhaus P, Nüssler AK. Effects of human hepatocyte growth factor on the proliferation of human hepatocytes and hepatocellular carcinoma cell lines. Eur Surg Res. 2004;36:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Monvoisin A, Neaud V, De Lédinghen V, Dubuisson L, Balabaud C, Bioulac-Sage P, Desmoulière A, Rosenbaum J. Direct evidence that hepatocyte growth factor-induced invasion of hepatocellular carcinoma cells is mediated by urokinase. J Hepatol. 1999;30:511-518. [PubMed] |
| 63. | Suzuki A, Hayashida M, Kawano H, Sugimoto K, Nakano T, Shiraki K. Hepatocyte growth factor promotes cell survival from fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppression. Hepatology. 2000;32:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1032] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 65. | Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 349] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 66. | Meyer DH, Bachem MG, Gressner AM. Modulation of hepatic lipocyte proteoglycan synthesis and proliferation by Kupffer cell-derived transforming growth factors type beta 1 and type alpha. Biochem Biophys Res Commun. 1990;171:1122-1129. [PubMed] |
| 67. | Roth S, Schurek J, Gressner AM. Expression and release of the latent transforming growth factor beta binding protein by hepatocytes from rat liver. Hepatology. 1997;25:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Giannelli G, Villa E, Lahn M. Transforming growth factor-β as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014;74:1890-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 69. | Wright JH, Johnson MM, Shimizu-Albergine M, Bauer RL, Hayes BJ, Surapisitchat J, Hudkins KL, Riehle KJ, Johnson SC, Yeh MM. Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: evidence for stromal induction of hepatocellular carcinoma. Int J Cancer. 2014;134:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | van Zijl F, Mair M, Csiszar A, Schneller D, Zulehner G, Huber H, Eferl R, Beug H, Dolznig H, Mikulits W. Hepatic tumor-stroma crosstalk guides epithelial to mesenchymal transition at the tumor edge. Oncogene. 2009;28:4022-4033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 71. | Ding S, Chen G, Zhang W, Xing C, Xu X, Xie H, Lu A, Chen K, Guo H, Ren Z. MRC-5 fibroblast-conditioned medium influences multiple pathways regulating invasion, migration, proliferation, and apoptosis in hepatocellular carcinoma. J Transl Med. 2015;13:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 906] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 73. | Park SY, Jeong KJ, Panupinthu N, Yu S, Lee J, Han JW, Kim JM, Lee JS, Kang J, Park CG. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 2011;30:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 74. | Wang DS, Dou KF, Li KZ, Song ZS. Enhancement of migration and invasion of hepatoma cells via a Rho GTPase signaling pathway. World J Gastroenterol. 2004;10:299-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Camps JL, Chang SM, Hsu TC, Freeman MR, Hong SJ, Zhau HE, von Eschenbach AC, Chung LW. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci USA. 1990;87:75-79. [PubMed] |
| 76. | Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1220] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 77. | Albrengues J, Bourget I, Pons C, Butet V, Hofman P, Tartare-Deckert S, Feral CC, Meneguzzi G, Gaggioli C. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014;7:1664-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 78. | Satoyoshi R, Kuriyama S, Aiba N, Yashiro M, Tanaka M. Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene. 2015;34:650-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Albrengues J, Bertero T, Grasset E, Bonan S, Maiel M, Bourget I, Philippe C, Herraiz Serrano C, Benamar S, Croce O. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat Commun. 2015;6:10204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 80. | Gopalakrishnan S, Van Emburgh BO, Robertson KD. DNA methylation in development and human disease. Mutat Res. 2008;647:30-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 81. | Procopio MG, Laszlo C, Al Labban D, Kim DE, Bordignon P, Jo SH, Goruppi S, Menietti E, Ostano P, Ala U. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol. 2015;17:1193-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 82. | Procopio MG, Laszlo C, Dotto GP. CSL-p53: From senescence to CAF activation. Cell Cycle. 2016;15:485-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1598] [Article Influence: 177.6] [Reference Citation Analysis (0)] |
| 84. | Sprinzl MF, Galle PR. Immune control in hepatocellular carcinoma development and progression: role of stromal cells. Semin Liver Dis. 2014;34:376-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Zhang HH, Mei MH, Fei R, Liu F, Wang JH, Liao WJ, Qin LL, Wei L, Chen HS. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat. 2010;17 Suppl 1:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 722] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 87. | Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 388] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 88. | Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 89. | Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 90. | Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1031] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 91. | Müller G. Dynamics of plasma membrane microdomains and cross-talk to the insulin signalling cascade. FEBS Lett. 2002;531:81-87. [PubMed] |
| 92. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2563] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 93. | Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1658] [Article Influence: 87.3] [Reference Citation Analysis (2)] |
| 94. | Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T, Taketomi A. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 95. | Mano Y, Aishima S, Fujita N, Tanaka Y, Kubo Y, Motomura T, Taketomi A, Shirabe K, Maehara Y, Oda Y. Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiology. 2013;80:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 96. | Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 97. | Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, Tai Y, Peng YW, Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 98. | Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 783] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 99. | Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, Stolz DB, Chen L, Fung JJ, Lu L. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 100. | Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015-3029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1346] [Cited by in RCA: 1614] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 101. | Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 270] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 103. | Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 104. | Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, Vizzutti F, Anania FA, Milani S, Rombouts K. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 105. | Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 106. | Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology. 2000;31:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Gressner AM, Lahme B, Meurer SK, Gressner O, Weiskirchen R. Variable expression of cystatin C in cultured trans-differentiating rat hepatic stellate cells. World J Gastroenterol. 2006;12:731-738. [PubMed] |
| 108. | Novo E, Cannito S, Zamara E, Valfrè di Bonzo L, Caligiuri A, Cravanzola C, Compagnone A, Colombatto S, Marra F, Pinzani M. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170:1942-1953. [PubMed] |
| 109. | Torimura T, Sata M, Ueno T, Kin M, Tsuji R, Suzaku K, Hashimoto O, Sugawara H, Tanikawa K. Increased expression of vascular endothelial growth factor is associated with tumor progression in hepatocellular carcinoma. Hum Pathol. 1998;29:986-991. [PubMed] |
| 110. | Jo M, Nishikawa T, Nakajima T, Okada Y, Yamaguchi K, Mitsuyoshi H, Yasui K, Minami M, Iwai M, Kagawa K. Oxidative stress is closely associated with tumor angiogenesis of hepatocellular carcinoma. J Gastroenterol. 2011;46:809-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 111. | Naylor MS, Stamp GW, Davies BD, Balkwill FR. Expression and activity of MMPS and their regulators in ovarian cancer. Int J Cancer. 1994;58:50-56. [PubMed] |
| 112. | Ding W, You H, Dang H, LeBlanc F, Galicia V, Lu SC, Stiles B, Rountree CB. Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology. 2010;52:945-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 113. | Elrick LJ, Leel V, Blaylock MG, Duncan L, Drever MR, Strachan G, Charlton KA, Koruth M, Porter AJ, Wright MC. Generation of a monoclonal human single chain antibody fragment to hepatic stellate cells--a potential mechanism for targeting liver anti-fibrotic therapeutics. J Hepatol. 2005;42:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 464] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 115. | Osawa Y, Oboki K, Imamura J, Kojika E, Hayashi Y, Hishima T, Saibara T, Shibasaki F, Kohara M, Kimura K. Inhibition of Cyclic Adenosine Monophosphate (cAMP)-response Element-binding Protein (CREB)-binding Protein (CBP)/β-Catenin Reduces Liver Fibrosis in Mice. EBioMedicine. 2015;2:1751-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 116. | Kubo N, Saito R, Hamano K, Nagasawa M, Aoki F, Takei I, Umezawa K, Kuwano H, Kojima I. Conophylline suppresses hepatic stellate cells and attenuates thioacetamide-induced liver fibrosis in rats. Liver Int. 2014;34:1057-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Weng TC, Shen CC, Chiu YT, Lin YL, Huang YT. Effects of armepavine against hepatic fibrosis induced by thioacetamide in rats. Phytother Res. 2012;26:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 118. | Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G137-G144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 119. | Tsai MK, Lin YL, Huang YT. Effects of salvianolic acids on oxidative stress and hepatic fibrosis in rats. Toxicol Appl Pharmacol. 2010;242:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 120. | Wang X, Ikejima K, Kon K, Arai K, Aoyama T, Okumura K, Abe W, Sato N, Watanabe S. Ursolic acid ameliorates hepatic fibrosis in the rat by specific induction of apoptosis in hepatic stellate cells. J Hepatol. 2011;55:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 121. | Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685-698. [PubMed] |
| 122. | Shu JC, He YJ, Lv X, Ye GR, Wang LX. Curcumin prevents liver fibrosis by inducing apoptosis and suppressing activation of hepatic stellate cells. J Nat Med. 2009;63:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 550] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 124. | Fischer R, Schmitt M, Bode JG, Häussinger D. Expression of the peripheral-type benzodiazepine receptor and apoptosis induction in hepatic stellate cells. Gastroenterology. 2001;120:1212-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Kim JY, Kim KM, Nan JX, Zhao YZ, Park PH, Lee SJ, Sohn DH. Induction of apoptosis by tanshinone I via cytochrome c release in activated hepatic stellate cells. Pharmacol Toxicol. 2003;92:195-200. [PubMed] |
| 126. | Ward SC, Waxman S. Fibrolamellar carcinoma: a review with focus on genetics and comparison to other malignant primary liver tumors. Semin Liver Dis. 2011;31:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 127. | Riehle KJ, Yeh MM, Yu JJ, Kenerson HL, Harris WP, Park JO, Yeung RS. mTORC1 and FGFR1 signaling in fibrolamellar hepatocellular carcinoma. Mod Pathol. 2015;28:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 128. | Huang L, Xu AM, Liu S, Liu W, Li TJ. Cancer-associated fibroblasts in digestive tumors. World J Gastroenterol. 2014;20:17804-17818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 129. | Steinbichler TB, Metzler V, Pritz C, Riechelmann H, Dudas J. Tumor-associated fibroblast-conditioned medium induces CDDP resistance in HNSCC cells. Oncotarget. 2016;7:2508-2518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 130. | Tanaka K, Miyata H, Sugimura K, Fukuda S, Kanemura T, Yamashita K, Miyazaki Y, Takahashi T, Kurokawa Y, Yamasaki M. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis. 2015;36:894-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 131. | Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2456] [Cited by in RCA: 2567] [Article Influence: 160.4] [Reference Citation Analysis (0)] |
| 132. | Hawsawi NM, Ghebeh H, Hendrayani SF, Tulbah A, Al-Eid M, Al-Tweigeri T, Ajarim D, Alaiya A, Dermime S, Aboussekhra A. Breast carcinoma-associated fibroblasts and their counterparts display neoplastic-specific changes. Cancer Res. 2008;68:2717-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 133. | Amornsupak K, Insawang T, Thuwajit P, O-Charoenrat P, Eccles SA, Thuwajit C. Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer. 2014;14:955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 134. | Yan H, Guo BY, Zhang S. Cancer-associated fibroblasts attenuate Cisplatin-induced apoptosis in ovarian cancer cells by promoting STAT3 signaling. Biochem Biophys Res Commun. 2016;470:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |