Copyright
©The Author(s) 2016.
World J Gastroenterol. Jul 28, 2016; 22(28): 6509-6519
Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6509
Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6509
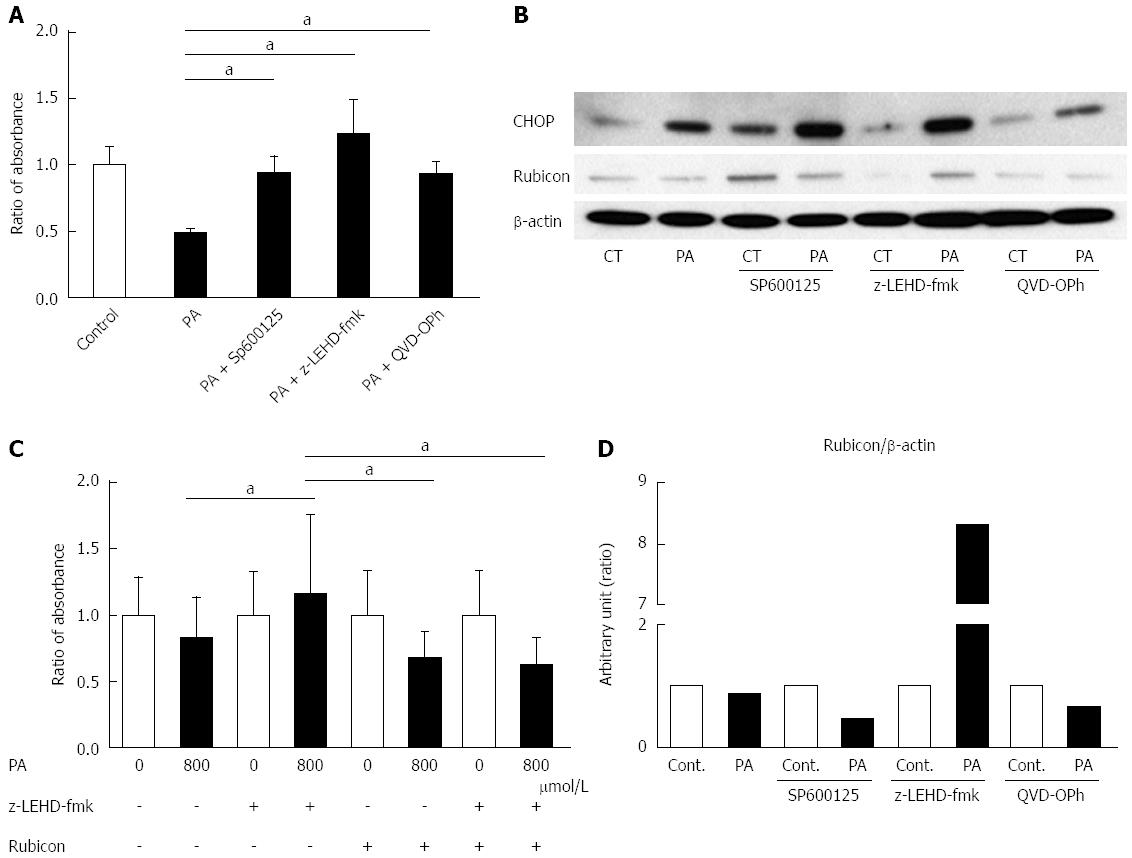
Figure 3 c-Jun N-terminal kinase inhibitor, Caspase-9 inhibitor, and Pan-Caspase inhibitor ameliorate palmitate-induced cell death, and Caspase-9 inhibition attenuates the decrease of Rubicon protein.
A: AML12 cells were incubated with 800 μmol/L PA for 10 h in the presence or absence of either 30 μmol/L SP600125, 20 μmol/L LEHD-fmk, or 20 μmol/L QVD-OPh. Untreated AML12 cells were used as the control. PA cytotoxicity was evaluated by cell proliferation assay. Living cells are presented as the ratio of the absorbance at the indicated conditions to that of AML12 without PA treatment; B: Whole cell lysates were prepared from AML12 cells treated with PA (800 μmol/L) for 10 h in the presence or absence of either 30 μmol/L SP600125, 20 μmol/L z-LEHD-fmk, or 20 μmol/L QVD-OPh. Immunoblot analysis of CHOP and Rubicon. β-actin was used as the loading control; C: The size of the AML12 cells after PA treatment (800 μmol/L) for 10 h was compared to that of untreated AML12 cells using the Image J software program. z-LEHD-fmk and/or siRubicon were used for Caspase-9 inhibition or Rubicon knockdown. The difference in cell size under each treatment condition is presented as the ratio to the cell size of the respective controls. All of the above experiments were repeated three times and representative results are shown. The quantitative data are presented as the mean ± SD; aP < 0.05.
- Citation: Suzuki A, Kakisaka K, Suzuki Y, Wang T, Takikawa Y. c-Jun N-terminal kinase-mediated Rubicon expression enhances hepatocyte lipoapoptosis and promotes hepatocyte ballooning. World J Gastroenterol 2016; 22(28): 6509-6519
- URL: https://www.wjgnet.com/1007-9327/full/v22/i28/6509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i28.6509