Published online May 28, 2016. doi: 10.3748/wjg.v22.i20.4946
Peer-review started: March 7, 2016
First decision: April 1, 2016
Revised: April 13, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: May 28, 2016
Processing time: 74 Days and 14.5 Hours
AIM: To provide an update on colorectal cancer (CRC) screening programmes in non-European Union (EU)-28 Council of Europe member states as of December 2015.
METHODS: The mission of the Council of Europe is to protect and promote human rights in its 47 member countries. Its 19 non-EU member states are Albania, Andorra, Armenia, Azerbaijan, Bosnia and Herzegovina, Republika Srpska, Georgia, Iceland, Liechtenstein, Republic of Moldova, Monaco, Montenegro, Norway, Russian Federation, San Marino, Serbia, Switzerland, FYR of Macedonia, Turkey, and Ukraine (EU-19). The main data source were GLOBOCAN, IARC, WHO, EUCAN, NORDCAN, ENCR, volume X of the CI5, the ministerial and Public Health Agency websites of the individual countries, PubMed, EMBASE, registries of some websites and the www.cochranelibrary.com, Scopus, www.clinicaltrials.gov, www.clinicaltrialsregister.eu, Research gate, Google and data extracted from screening programme results.
RESULTS: Our results show that epidemiological data quality varies broadly between EU-28 and EU-19 countries. In terms of incidence, only 30% of EU-19 countries rank high in data quality as opposed to 86% of EU-28 states. The same applies to mortality data, since 52% of EU-19 countries as against all EU-28 countries are found in the high ranks. Assessment of the method of collection of incidence data showed that only 32% of EU-19 countries are found in the top three quality classes as against 89% of EU-28 countries. For the mortality data, 63% of EU-19 countries are found in the highest ranks as opposed to all EU-28 member states. Interestingly, comparison of neighbouring countries offering regional screening shows, for instance, that incidence and mortality rates are respectively 38.9 and 13.0 in Norway and 29.2 and 10.9 in Sweden, whereas in Finland, where a national organised programme is available, they are respectively 23.5 and 9.3.
CONCLUSION: Cancer screening should be viewed as a key health care tool, also because investing in screening protects the weakest in the population, decreases the social burden of cancer, and reduces all types of health care costs, including those for radical surgery, long-term hospitalisation, and chemotherapy.
Core tip: In the WHO Europe Region, colorectal cancer (CRC) is the first tumour with 471000 new cases per year and a mortality rate of 28.2 per 100000 population. Large-scale studies have found a reduction in mortality due to the adoption of population-based screening programmes. A 2010 European Parliament resolution called for the adoption of prevention programmes. As a result, some member states have begun enacting programmes, others are organising strategies for CRC screening implementation, and others still are moving from pilot projects to national-scale programmes. The present systematic review provides an update on CRC screening programmes in non EU-28 European Council States.
- Citation: Altobelli E, D’Aloisio F, Angeletti PM. Colorectal cancer screening in countries of European Council outside of the EU-28. World J Gastroenterol 2016; 22(20): 4946-4957
- URL: https://www.wjgnet.com/1007-9327/full/v22/i20/4946.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i20.4946
Although cervical, breast and colorectal cancer are the only tumours for which screening has proven efficacy and cost-effectiveness, in several European countries screening implementation is fraught with difficulties. This is especially true of programmes regarding colorectal cancer (CRC)[1-3], a highly common malignancy. According to GLOBOCAN data[3], 1.36 million new cases affecting 17.2 per 100000 population (746000 men and 614000 women) are diagnosed in the world each year, and 693000 people (373000 men and 320000 women) die from CRC, accounting for a yearly mortality rate of 8.4 per 100000.In the World Health Organisation (WHO) Europe Region, CRC is the first tumour by incidence, with 471000 new cases each year and a mean mortality rate of 28.2 per 100000 population[4]. In the European Union (EU-28), its mean incidence rate is 31.3 per 100000 population, with 345000 new cases per year and an incidence per 100000 population of 39.5 for men 39.5 and 24.4 for women. The mean CRC incidence rates for men and women in the WHO Europe Region are 35.6 and 22.6 per 100000 population, respectively. In addition, with 228000 deaths per year and a mortality rate of 12.3 per 100000 population, CRC is the second cause of cancer death after lung cancer for men and women in the region[4]. The mean mortality rates per 100000 population in EU-28 countries and the WHO Europe Region are respectively 15.2 and 15.7 for men and 9.0 and 9.7 for women[4].
CRC incidence is quite variable in EU-28 countries, and is higher in central and northern member states than in eastern ones. However, the lower rates found in eastern Europe are higher than the world mean[3]. This has prompted the Council of Europe to recommend the priority activation of CRC screening programmes[5]. According to a 2008 European Commission report on the diffusion of CRC screening programmes in the EU, only 12 of the then 22 member states had population-based screening programmes; the others were recommended to provide to their citizens equal access to cancer prevention[6].
Crucially, more than 95% of CRC cases could benefit from surgical treatment if diagnosed early[7]. Several large-scale studies have found a considerable reduction in mortality due to the adoption of population-based screening programmes[8,9].
The first European guidelines on CRC screening and the quality of CRC diagnosis were issued in 2010[10]. A European Parliament resolution of 6 May 2010 asked the Commission to promote the adoption of prevention programmes by any means and to encourage member states to allocate further resources to primary prevention and early diagnosis through screening[11]. As a result, some member states have begun enacting programmes, others are organising strategies for CRC screening implementation[3], and others still are moving from pilot projects to national-scale programmes[12-16].
The aim of the present systematic review is to provide an update on CRC screening programmes in non EU-28 European Council member states as of December 2015.
The Council of Europe is a supranational institution founded in 1949 by the Treaty of London. Its mission is to protect and promote human rights in member countries. There are 47 member countries and a number of states with observer status. All EU-28 States are members (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, the Netherlands, and the United Kingdom). The other 19 countries (hereafter EU-19) are in the European area: Albania, Andorra, Armenia, Azerbaijan, Bosnia and Herzegovina, Republika Srpska, Georgia, Iceland, Liechtenstein, Republic of Moldova, Monaco, Montenegro, Norway, Russian Federation, San Marino, Serbia, Switzerland, FYR of Macedonia, Turkey, and Ukraine.
The main data source was the GLOBOCAN 2012 website of the International Agency for Research on Cancer (IARC), which provides access to several databases that enable assessing the impact of CRC in 184 countries or territories in the world[4].
Additional sources were the WHO, EUCAN and NORDCAN, the European Network of Cancer Registries (ENCR), volume X of the CI5, and the ministerial and Public Health Agency websites of the individual countries. The PubMed search used “Early Detection of Cancer” or “Colorectal Cancer screening” AND “state name” for each of the 19 countries. A MeSH search was conducted using the same criteria. The EMBASE did not provide further relevant results. The registries of some websites and the http://www.cochranelibrary.com, Scopus, http://www.clinicaltrials.gov, http://www.clinicaltrialsregister.eu, Research gate, and Google databases were also consulted. Other data were extracted from screening programme results.
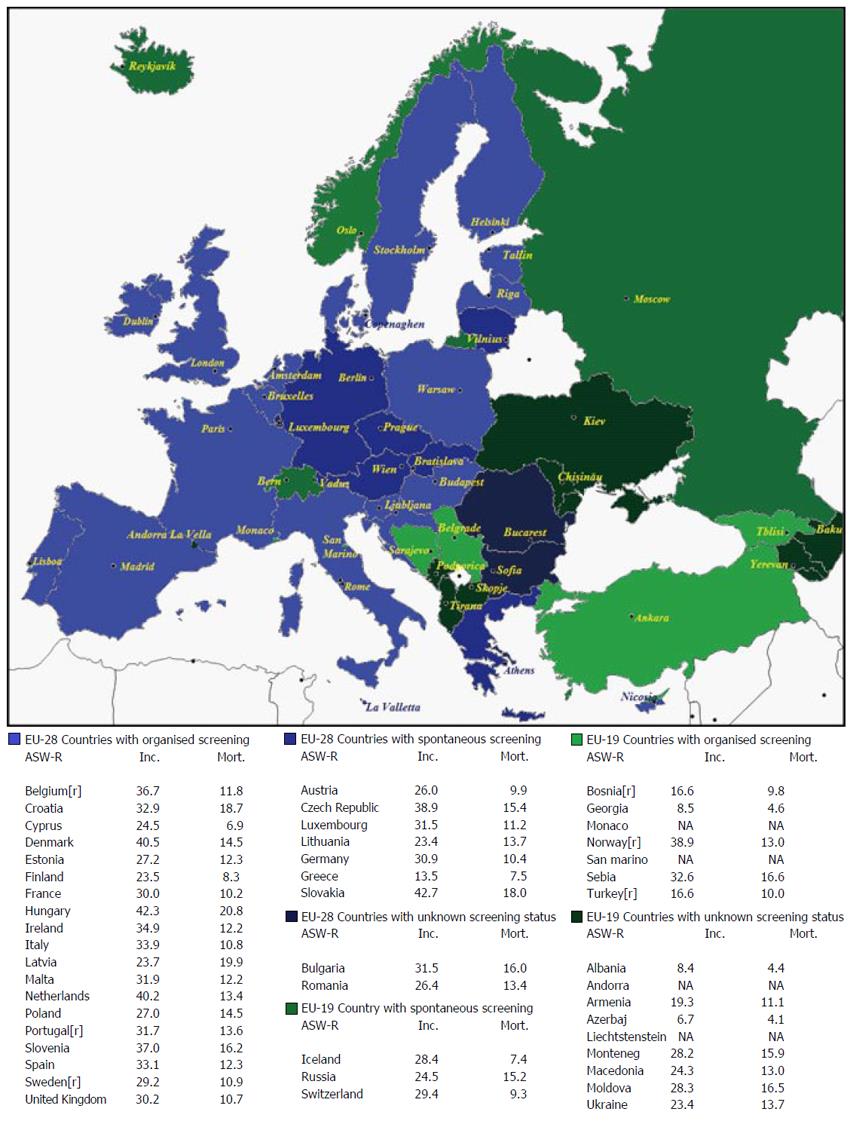
Incidence and mortality data, their age-standardised rates per 100000 population (ASR-W), and 5-year prevalence estimates for 2012 are reported by gender in Table 1. The quality of incidence and mortality data of EU-19 and EU-28 based on Data Sources and Methods[17] is compared in Table 2. The information regarding screening programmes in EU-19 is shown in Table 3. Finally mean income, total population, the existence of any registries, the availability of early detection tests at the public primary health care level, and the ranking of CRC incidence and mortality in EU-19 countries are reported in Table 4. The distribution of screening programmes (organised, spontaneous, unknown) in EU-28 and EU-19 countries is shown in Figure 1.
| Country | WOMAN | MAN | ||||||||||
| Incidence | 5-yr prevalence | Mortality | Incidence | 5-yr prevalence | Mortality | |||||||
| Cases | ASR (W) | Cases | % | Cases | ASR (W) | Cases | ASR (W) | Cases | % | Cases | ASR (W) | |
| Albania | 167 | 7.9 | 501 | 39.9 | 90 | 4.0 | 175 | 9.0 | 526 | 42.0 | 97 | 4.8 |
| Andorra | NR | |||||||||||
| Armenia | 4.4 | 17.0 | 939 | 68.6 | 281 | 9.7 | 426 | 22.8 | 855 | 77.1 | 261 | 13.4 |
| Azerbaijan | 346 | 6.4 | 209 | 4.0 | 755 | 19.8 | 313 | 7.1 | 661 | 18.5 | 187 | 4.3 |
| Bosnia and Herzegovina | 489 | 13.3 | 1427 | 84.8 | 327 | 7.7 | 620 | 20.7 | 1811 | 119.1 | 422 | 12.7 |
| Georgia | 300 | 7.5 | 608 | 31.3 | 173 | 4.0 | 305 | 9.9 | 631 | 38.3 | 177 | 5.5 |
| Iceland | 79 | 28.3 | 238 | 183.6 | 21 | 5.8 | 78 | 28.9 | 232 | 177.5 | 27 | 9.3 |
| Liechtenstein | NR | |||||||||||
| Republic of Moldova | 716 | 23.0 | 1736 | 111.0 | 409 | 12.6 | 799 | 36.0 | 1963 | 143.2 | 491 | 22.0 |
| Monaco | NR | |||||||||||
| Montenegro | 107 | 21.1 | 314 | 118.7 | 67 | 12.0 | 157 | 36.2 | 465 | 187.3 | 95 | 20.7 |
| Norway | 1947 | 35.8 | 5665 | 279.6 | 779 | 12.1 | 1966 | 42.6 | 5839 | 289.8 | 727 | 14.3 |
| Russian Fed | 33183 | 21.8 | 78454 | 119.1 | 21791 | 12.7 | 26745 | 30.0 | 63572 | 116.4 | 18116 | 19.9 |
| San Marino | NR | |||||||||||
| Serbia | 2143 | 23.3 | 6281 | 151.4 | 1213 | 11.5 | 3370 | 43.4 | 9919 | 248.8 | 1922 | 22.8 |
| Switzerland | 2167 | 23.6 | 6522 | 193.8 | 718 | 6.4 | 2707 | 36.3 | 8340 | 259.9 | 1668 | 12.8 |
| FYR Macedonia' | 366 | 20.5 | 1070 | 124.0 | 213 | 10.8 | 421 | 28.4 | 1256 | 147.3 | 239 | 15.5 |
| Turkey | 5041 | 13.1 | 10690 | 38.2 | 3030 | 7.8 | 6889 | 20.6 | 14982 | 54.7 | 4128 | 12.6 |
| Ukraine | 9780 | 19.9 | 23110 | 109.6 | 5704 | 10.8 | 6269 | 29.9 | 22120 | 127.8 | 5929 | 18.8 |
| EU-28 | 151920 | 24.4 | 417252 | 189.0 | 69087 | 9.0 | 193426 | 39.5 | 535845 | 257.8 | 82959 | 15.2 |
| Data source | |||
| EU-19 | EU-19 | EU-28 | EU-28 |
| Incidence | Mortality | Incidence | Mortality |
| A: Iceland Norway, Ukraine | 1: Iceland, Republic of Moldova | A: Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Ireland, Latvia, Lithuania, Malta, Sweden, Slovenia,Slovakia, The Netherlands, United Kingdom, Finland, France (Martinique) | 1: Estonia, Hungary, Ireland, Latvia, Lithuania, Malta, Slovenia, Slovakia, Romania, United Kingdom, Finland |
| B: Serbia, Switzerland | 2: Azerbaijan, Norway, Russian Federation, Serbia, Switzerland | ||
| C: Turkey | 2: Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, France, Germany, Italy, Luxembourg, Spain, Sweden, The Netherlands, France (Guadalupe), (La Reunion), France (Martinique), France (Guiana) | ||
| D: Bosnia Herzegovina, Russian Federation | B: France, Germany, Italy, Spain | ||
| 3: Albania, Armenia, FYR Macedonia | C: Portugal, Poland | ||
| E: - | D: Luxembourg, France (La Reunion) | ||
| F: - | 4: - | E: Romania | |
| G: Albania, Armenia, Azerbaijan, FYR Macedonia, Georgia, Montenegro, Republic of Moldova | 5: Bosnia Herzegovina | F: - | |
| 6: Georgia, Montenegro, Turkey | G: Greece, Hungary, France (Guadalupe), France (Guiana) | ||
| 3: Greece, Portugal, Poland | |||
| 4: - 5: - 6: - | |||
| Methods | |||
| EU-19 | EU-28 | EU-19 | EU-28 |
| Incidence | Mortality | Incidence | Mortality |
| 1: Iceland, Norway | 1: Albania, FYR Macedonia, Iceland, Norway, Republic of Moldova, Serbia, Switzerland | 1: Austria, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, Germany, Ireland, Latvia, Lithuania, Malta, Slovakia, Slovenia, Sweden The Netherlands, United Kingdom, France (La Reunion), France (Martinique) | 1: Austria. Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France (metropolitan) Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Poland, Portugal, Slovakia, Slovenia, Spain, Sweden, The Netherlands, United Kingdom, France (Martinique) |
| 2: Bosnia Herzegovina, Ukraine | |||
| 3: Turkey, Switzerland | 2: Armenia, Azerbaijan, Bosnia Herzegovina, Georgia, Ukraine | ||
| 4: Albania, FYR Macedonia, Republic of Moldova, Serbia | |||
| 2: Belgium, Cyprus | |||
| 3: - 4: - | 3: France (metropolitan) , Germany, Italy, Poland, Spain | ||
| 5: Armenia, Azerbaijan, Georgia | 5: Turkey | ||
| 6: Montenegro | 4: Greece, Hungary, Luxembourg, Portugal | ||
| 6: Turkey | 5: Romania, France (Guadalupe), | 2: Belgium, Cyprus, France (Guadalupe) | |
| 7: - 8: - | 6: - 7: - 8: - 9: - | ||
| 9: Montenegro | 3: Greece, Portugal, Poland | ||
| 4: - | |||
| 5: France (La Reunion) | |||
| 6: - | |||
| Country | Program | Test | Screening interval (yr) | Age (yr) | Program start | Pop target | Level of participation (%) | ||
| Type | Status | Region | |||||||
| Federation of Bosnia and Heregovina[22] | Spontaneous | NatW | All country | FOBT | - | > 50 | - | - | - |
| Organised | |||||||||
| Republika Srpska[22] | Spontaneous | NatW | All country | FOBT | - | > 50 | - | - | - |
| Georgia[51] | Organised | NatW | Tblisi | gFOBT | 2 | 50-69 | - | 25388 | 53 |
| OutsideTblisi | gFOBT | 2 | 50-69 | - | 71364 | 84 | |||
| Iceland[18] | Programmed as organised | NatW | All country | FOBT | 2 | 55-75[19] | - | 86000[19] | |
| Iceland[19] | Spontaneous | NatW | All country | Colonoscopy | 50-59 | 30 | |||
| Monaco[35] | PB | Natw | All country | iFOBT | 2 | 50-80 | 2006 | 16000[35] | 60 |
| (from 2015) | |||||||||
| gFOBT | |||||||||
| Montenegro[22,28] | None | - | - | - | - | - | - | - | - |
| Montenegro[28] | Not PB | PilotStudy | Danilograv, municipality of Podgorica | iFOBT | - | 50-74 | 2010-2011 | 4500 | 33.3 |
| Norway[20] | PB | Pilot Study | Østfold, Akershus and Buskerud | iFOBT | 2 | 2012 | |||
| Norway[21] | PB | Pilot Study RCT | Oslo and Telemark in 1999-2001 NORCAPP | FOBT and FOBT + Sigmoidoscopy | - | 55-64 | 1999-2000 | 13823 | 64.8 |
| Russian Fed[42] | PB | Pilot Study | Sant Petersburb, all 18 town district | iFOBT | 48-75 | November 15 | 20000 | ||
| Russian Fed[43] | NPB | Pilot Study | Kazan, Tatarstan Republic | FOBT, DRE, questionnaire | - | 2010 | 1071 | ||
| San Marino[36,37] | PB | Natw | All country | iFOBT | 2 | 50-79 | 2009 | 65[37] | |
| Serbia[31] | PB | Natw | All country | iFOBT | 2 | 50-74 | 789330 | 58.38 | |
| Switzerland[32] | Spontaneous | NatW | All country | FOBT or Colonoscopy | 2, 10 | 50-80 | 2013 | 13170 | 22 |
| Switzerland[33] | PB | Pilot Study RCT | Glarus, Vallée du Joux Uri | FOBT and or Colonoscopy | - | 50-80 | 2001 | 20000 | |
| Switzerland[34] | PB | Pilot Study, NRCT | Vaud | iFOBT or Colonoscopy | 50-69 | 2015 | |||
| Turkey[44] | Organised | NatW | FOBT | 50-69 | 2009 | 11681513 | 30[44] | ||
| Spontaneous | |||||||||
| Ukraine[46,54] | PB | NatW | Not available | Not available | Not available | 2002-2006 | Not available | ||
| Country | Income1 | Total population | Cancer registry | Availability at public primary health care levels of early detection tests | Ranking CRC incidence2 | Ranking CRC mortality2 | |||
| Faecal occult blood test | Bowel cancer screening by exam or colonoscopy | Man | Woman | Man | Woman | ||||
| North Europe | |||||||||
| Iceland2 | High | 326000 | National, population-based | - | - | 3rd | 3rd | 2nd | 4th |
| Norway2 | High | 4994000 | National, population-based | Yes | - | 2nd | 2nd | 3rd | 2nd |
| Central Europe | |||||||||
| Liechtenstein3 | High non OECD | 36925 | NA | - | - | 2nd | 3rd | NR | NR |
| Monaco | High non OECD | 38000 | Hospital-based | Yes | Yes | NR | NR | NR | NR |
| Switzerland3 | High | 7997000 | Sub-national population-baseda | Yes | Yes | 2nd | 2nd | 3rd | 3rd |
| South Europe | |||||||||
| Andorra | High non OECD | 78000 | Hospital-based | Yes | Yes | NR | NR | NR | NR |
| San Marino | High non OECD | 31000 | National population-based | Yes | Yes | NR | NR | NR | NR |
| Balcanian countries | |||||||||
| Albania3 | Upper middle | 3162000 | Sub-national, hospital-based | - | - | > 5th | 5th | > 5th | 5th |
| Bosnia and Herzegovina3 | Upper middle | 3834000 | NA | - | - | 3rd | 2nd | 2nd | 3rd |
| Montenegro3 | Upper middle | 621000 | NA | - | - | 2nd | 2nd | 2nd | 3rd |
| Serbia3 | Upper middle | 9553000 | Sub-national | Yes | Yes | 2nd | 2nd | 2nd | 3rd |
| FYR of Macedonia3 | Upper middle | 2106000 | National | Yes | - | 3rd | 3rd | 2nd | 2nd |
| Eastern Europe | |||||||||
| Republic of Moldova3 | Lower middle | 3514000 | National, hospital-based | Yes | Yes | 2nd | 2nd | 2nd | 2nd |
| Russian Fed3 | High non OECD | 143000000 | Sub-national, population-basedb | Yes | Yes | 3rd | 2nd | 3rd | 2nd |
| Turkey3 | Upper middle | 73997000 | Sub-national population-basedc | Yes | Yes | 4th | 3rd | 4th | 3rd |
| Ukraine3 | Lower middle | 45530000 | National, population-based | - | - | 2nd | 2nd | 2nd | 2nd |
| Caucasican countries | |||||||||
| Armenia3 | Lower middle | 2969000 | National, hospital-based | Yes | - | 5th | 3rd | 4th | 2nd |
| Azerbaijan3 | Upper middle | 9309000 | NA | Yes | - | 4th | 4th | 5th | 5th |
| Georgia3 | Lower middle | 4358000 | Sub-national population-based | - | - | 5th | > 5th | 5th | > 5th |
The results of the present systematic review are listed by physical geographical area as well as disaggregated by state. The incidence and mortality data are reported as ASR-W per 100000 population.
The only North European countries that are not also EU-28 members are Iceland and Norway. The United Kingdom and Northern Ireland, Ireland, Finland, Denmark, Estonia, and Latvia offer organised national screening programmes and Sweden an organised regional programme; only Lithuania adopts spontaneous screening (Figure 1).
Iceland: The incidence rate of CRC in Iceland is 28.9 and 28.3 in men and women, respectively, with a mortality rate of 9.3 for men and 5.8 for women (Table 1).The national cancer registry, linked to the NORDCAN project, covers the whole population and provides high-quality data (Table 2). Iceland has no active organised CRC screening programme (Table 3). The decision to adopt one, made in 2008[18], was postponed due to the economic crisis. According to a recent congress communication[19], a programme offering screening with the iFOBT at 2-year intervals to 55 to 75 year olds is due to start soon (Table 3). Until then, only spontaneous screening with the iFOBT will be available at the level of public primary health care (Table 4). CRC is the third most common tumour in both genders in the country and the fourth and second cause of cancer death in Iceland (Table 4).
Norway: In this country the incidence of CRC is 42.6 among men and 35.8 among women, with a mortality rate - 12.1 in men and 14.3 in women (Table 1). High data quality is ensured by a national cancer registry linked to the NORDCAN that covers the whole population (Table 2). A pilot study offering the iFOBT at 2-year intervals was activated in 2012 in the Ostfold region[20]. In a randomised controlled study (NORCCAPP) conduced in the Oslo and Telemark areas in 1999-2001 the population was assigned to three groups that were tested with the iFOBT, received the iFOBT + sigmoidoscopy, or were just asked to report if they had had a diagnosis of CRC in the course of the study[21] (Table 3). CRC is the second most common tumour in both sexes and the second cause of cancer death for both sexes in Norway (Table 4).
Several of these countries are EU-19 States: Albania, Republika Srpska, Bosnia and Herzegovina, Montenegro, and Serbia. Slovenia and Croatia are EU-28 Member states offering organised screening programmes (Figure 1).
Albania: Albania has a low CRC incidence rate, 9.0 among men and 7.9 among women, and an equally low mortality rate, respectively 4.8 and 4.0 (Table 1). Hospital-based disease registries provide non-excellent data quality (Table 2). Neither spontaneous nor organised screening is available[22]. The most recent data are for 2011. A 2015 paper[23] that first measured the frequency of gastrointestinal polypoid lesions in the Albanian population stressed the absence of a screening programme. According to the WHO report[24], neither the FOBT nor colonoscopy are available at the level of public primary health care (Table 4).
Bosnia and Herzegovina, Republika Srpska: In the Federation of Bosnia and Herzegovina the incidence of CRC is 20.7 among men and 13.3 among women, with a mortality rate of 12.7 in men and 7.7 in women. Data quality is not excellent (Table 2). According to Giordano et al[22], spontaneous and organised screening based on the FOBT is available for those aged more than 50 years. However, Buturovic reports that in the Konjic area colonoscopy is not available[25]. As shown in Table 4, the WHO has no data on the availability of screening tests (FOBT, colonoscopy) at the level of public primary health care[26]. The tumour represents the third and second most common cancer and the second and third cause of death in the country (Table 4).
In the Republika Srpska only spontaneous screening is available to subjects older than 50 years[22]. Again, there is no clear information on screening programmes.
FYR Macedonia: In this country CRC incidence is moderately high in men (28.4) as well as women (20.5) (Table 1) and mortality rates of 15.5 and 10.8, respectively. Data quality is mediocre (Table 2). There seem to be no organised screening programmes, even though the iFOBT is available at the public primary health care level[27] (Table 4). CRC is the third most common tumour in the country for both sexes and the second cause of cancer death (Table 4).
Montenegro: The incidence of CRC in Montenegro is 36.2 among men and 21.1 among women, with a mortality rate of 20.7 in men and 12.0 in women. Data quality is poor (Table 2). A population-based screening programme using the iFOBT and involving subjects aged 50 to 74 years was conducted from February 2010 to March 2011 in Danilograv municipality (Podgorica)[28], while neither organised nor opportunistic screening is available in the other areas[22]. According to WHO data (Table 4), early detection tests are not available at the public primary health care level[29]. CRC ranks respectively as the second and third cause of cancer death in Montenegro (Table 4).
Serbia: At 43.3 in men and 23.3 in women, the incidence of CRC in Serbia is fairly high and the tumour is the second most common malignancy in both sexes. The mortality rates are 28.8 in men and 11.5 in women, and CRC is respectively the second and third cause of cancer death in the country. Data quality is good (Table 2). In 2013 Serbia implemented a national screening programme by extending a programmes that had been active in Vozdovac, Subotica and Zrenjanin since 2005[30]. The current programme is offered to 50 to 74 year olds without evidence of CRC and uses the iFOBT. Its results are available online. The rate of participation as of 30 September 2015 was 58.38%[31].
France, Poland, Hungary, and the Netherlands offer national organised programmes, and Belgium a regional programme. Austria, Germany, the Czech Republic, Slovakia, and Luxembourg provide for spontaneous screening (Figure 1). The other countries in the area are Switzerland, Liechtenstein, and the Principality of Monaco.
Switzerland: The incidence rate of CRC is 36.3 in men and 23.3 in women; CRC is the second most common neoplasm in the country (Table 4). With a mortality rate of 28.8 in men and 6.4 in women, CRC ranks as the third cause of cancer death in both sexes (Table 4). Data quality is good and in line with that of neighbouring countries (Italy, France, and Germany). Since 2002, the Swiss Federal Statistical Office has been conducting a telephone survey, the Swiss Health Interview Survey (SHIS). In 2007, it assessed for the first time the date and reason for the use of the iFOBT and/or colonoscopy and asked detailed questions on screening[32]. The 2007 results found a rate of participation of 18.9% for both methods. In 2012 participation rose to 22.2% (P = 0.036)[33]; colonoscopy rose from 8.2% in 2007 to 15% (P < 0.001) and the iFOBT fell from 13% to 9.8% (P = 0.002). In 2007 the prevalence of CRC screening among respondents was 24.5% among higher-income respondents (> $6000 a month) and 10.5% in those with a low income (< $2000); the 2012 survey found a similar difference (respectively 28.6% and 16.0%). There was no association with education or occupation[32]. Since 1 July 2013 the test (colonoscopy every 10 years and iFOBT every 2 years) is partially covered by the mandatory insurance for those aged 50 to 69 years. In Uri Canton an organised programme is offered to 50-80 year olds without a past or current history of CRC. The programme was introduced in 2000 and the results of the first round have been published[33]; the patient could choose among colonoscopy, sigmoidoscopy, and iFOBT + sigmoidoscopy, and more than 70% opted for colonoscopy. Another programme in Vaud Canton offers screening to individuals aged 50 to 69 years having no risk factors or a past or current history of CRC; they can choose between the iFOBT every 2 years and colonoscopy every 10 years; the excess is paid for by the Cantonal administration, the remaining expenses are sustained by the patient. The programme is co-ordinated by the Fondation Vaudoise pour le Dépistage du Cancer[34].
Liechtenstein: In this tiny state the incidence data are provided by the National Bureau of Statistics. CRC is the second most common cancer in men and the third in women. No data are available on CRC screening programmes, neither through institutional websites nor through the WHO (Table 4).
Principality of Monaco: Official incidence and mortality data validated by the WHO are not available for this city-state, but they are probably similar to those of France. The Centre Monégasque de Dépistage has been coordinating the CRC screening campaign since 2006. The programme uses the iFOBT. Subjects receiving a letter of invitation can choose between picking up the examination kit at their general practitioner (GP) or at the screening centre. Those with a positive test are referred to the Centre Hospitalier Princesse Grace, where digestive endoscopy is performed to establish the cause of the bleeding. The service is offered to residents and foreign workers aged 50-80 years. Participation is about 60%. In March every year, the Blue March is organised in France and the Principality to promote CRC awareness and focus the attention of the population on the value of CRC prevention[35].
In this region, Italy, Spain, Malta, and Cyprus offer national organised programmes, and Portugal a regional organised programme. In Greece screening is spontaneous (Figure 1). There are also two tiny states, Andorra and San Marino.
San Marino: Official incidence and mortality data are not available; however, since San Marino is nestled in the Italian region of Emilia-Romagna, they should be similar to those of Italy. A national programme, offered to individuals aged 50 to 75 years and managed by the Screening Centre of the Istituto per la Sicurezza Sociale, has been in place since 2009. The test is delivered home by mail and the recipient is asked to take it to the relevant Health Centre. Failure to do so in three months results in a reminder. Subjects with a positive test are referred to the State Hospital for a second-level examination, usually colonoscopy[36]. Participation rates (65%) have been illustrated at a press conference and are encouraging[37].
Andorra: WHO incidence and mortality data are not available, but they can reasonably be considered to resemble those of Cataluña. According to the 2014 WHO report - Cancer Country Profiles, CRC screening with the iFOBT and colonoscopy are generally available at the public primary health care level in this small Pyrenean state[38] (Table 4). However, unlike the case of breast cancer, the institutional website provides no information on CRC prevention.
In this area, Romania and Bulgaria do not offer organised screening (Figure 1). The other states in the region include the Republic of Moldova, the Russian Federation, Turkey and Ukraine.
Republic of Moldova: In this country CRC is the second most common neoplasm in men and women alike (Table 4) with an incidence of 36.0 and 23.0 respectively. The mortality rate is 22.0 and 12.6, respectively; CRC is the second cause of cancer mortality in the country (Table 4). The literature provides no information on screening programmes, but the FOBT and colonoscopy are both available at the public primary health care level[39] (Table 4).
Russian Federation: CRC is the third tumour by incidence and mortality among men (respectively 30.0 and 19.9) and the second among women (respectively 21.8 and 11.5) (Tables 1 and 4). Data quality is mediocre (Table 2). No national screening programmes are in place[40]. The poor awareness regarding CRC involves a high rate of late diagnoses (25.6% in stage IV vs 18.8% in the United States) despite the fact that the iFOBT and colonoscopy are largely available at the public primary health care level[41] (Table 4). A screening campaign launched in November 2015 in the Saint Petersburg area, which hosts the sole cancer registry in the Federation[42], follows an earlier, small-scale programme set up in the Kazan region[43] (Table 3).
Turkey: In Turkey CRC incidence is 20.6 and 13.1 in men and women, respectively (Table 1). The mortality rate is 12.6 and 10.8, respectively (Table 1). Data quality is mediocre (Table 2). The national Cancer Control Department has been promoting cancer prevention campaigns since 2003. In 2009 there were no active population-based programmes, but merely some sporadic pilot studies[44]. However, a KETEM centre per province (including 2 in Istanbul and 3 in Ankara) supervise the execution of cancer screening (breast and cervical cancer) according with national guidelines; CRC was added in 2009. Screening is not covered by Social Security provisions and is sustained by the Health Ministry only for older and poorer people. Screening is largely spontaneous, but some centres like Ankara have started population-based programmes[44] (Table 3). In the future, GPs will be given the task of encouraging patients to pursue early CRC detection[44]. Both the FOBT and colonoscopy are available at the public primary health care level, as reported by the WHO country cancer profile[45]. CRC is the third and fourth cause of cancer death in the country (Table 4).
Ukraine: In Ukraine, the incidence rate of CRC is 29.9 in men and 19.9 in women and the tumour ranks second as a cause of cancer death. The mortality rate is 18.8 in men and 10.8 in women. Data quality is excellent (Table 2). According to the cancer registry, the 2002-2006 cancer plan included prevention programmes for cervical, breast, colorectum, prostate, skin, and oral cavity[46]. Based on WHO data, screening tests are not available at the public primary health care level[47] (Table 4).
These states lie on the extreme eastern boundary of Europe, where the Caucasus traditionally represents the geographical border with the Middle East.
Armenia: In Armenia CRC is the fifth most common tumour among men (22.8) and the third among women (17.0); it is the fourth cause of cancer death among men (13.4) and the second among women (9.7) (Tables 1 and 4). Data quality is fairly poor (Table 2). The literature supplies no information on screening, but the FOBT is available at the level of public primary health care[48].
Azerbaijan: In Azerbaijan CRC incidence is fairly low in both sexes (7.1 in men and 6.4 in women) and is the fourth most common tumour. CRC mortality is lower in men (4.3) than in women (19.8) and the tumour is the fifth cause of cancer death in the country (Tables 1 and 4). Data quality is poor (Table 2). Although there are no published data on screening, the WHO report indicates that the FOBT is available at the public primary health care level[49].
Georgia: In this country CRC incidence is low: 9.9 in men and 7.5 in women. The mortality figures are 5.5 in men and 4.0 in women. Data quality is poor (Table 2). Even though according to the WHO reports neither the FOBT nor colonoscopy is available for first-level screening[50], the literature and the official websites mention a CRC screening campaign and report its results[51]. These data are summarised in Table 2.
Improvements in the health status of populations and the progressive increase in life expectancy call for the promotion and diffusion of healthy lifestyles, to reduce the impact of non-communicable diseases; in particular, cancer entails an extremely high social and psychological burden. The chief mission of the Council of Europe is to promote and protect human rights in member states by issuing orientation papers and guidelines. In particular, art. 11 of the Social Charter, “The Right to Protection of Health”, explicitly mentions the promotion of the health of the citizens of member states[52] and art. 3 of the Convention on Human Rights and Biomedicine makes reference to equal and appropriate access to health care[53].
Interestingly, comparison of neighbouring countries offering regional screening shows, for instance, that incidence and mortality rates are 38.9 and 13.0 in Norway and 29.2 and 10.9 in Sweden, whereas in Finland, where a national organised programme is available, they are respectively 23.5 and 9.3[54].
Epidemiological data quality varies broadly between EU-28 and EU-19 countries. In terms of incidence, only 30% of EU-19 countries rank high in data quality (A, B and C), as opposed to 86% of EU-28 states. The same applies to mortality data: 52%of EU-19 countries as against all EU-28 countries are found in the high ranks (1, 2, or 3) (Table 2).
Assessment of the method of collection of incidence data showed that only 32% of EU-19 countries were found in the top three quality classes (A, B and C) as against 89% of EU-28 countries. For the mortality data, 63% of EU-19 countries were found in the highest ranks as opposed to all EU-28 member states (Table 2). The continuous improvement in the quality of epidemiological data collection critically supports public health decision-making, in that it represents the phenomena studied in an increasingly accurate manner in all geographical areas. This is all the more important at a time when the European continent is besieged by problems such as the economic downturn, climate change, international tensions and, last but definitely not least, the management of strong migration flows. The economic crisis has hampered the activation of large-scale screening programmes in countries, like Iceland[19], where the recession has had a devastating impact, involving their postponement. In other emerging states and areas, like Serbia[30,31], Georgia[51], and Saint Petersburg[42], population-based programmes have been implemented despite the crisis, making cancer prevention a priority, also to reduce social inequality. Clearly, it is critical to stimulate awareness of the importance of cancer prevention through screening.
In a recent British study, Moffat et al[55] found that awareness is crucial where cancer and its early detection are concerned. The authors measured CRC symptom knowledge before and after an awareness campaign directed at lower-income subjects, and showed that the campaign improved the knowledge of suspicious symptoms. Notably, the campaign also increased screening requests to GPs. Another key finding was a greater awareness of CRC among the elderly, suggesting that differential campaigns are not required for different age classes. Large-scale campaigns like the Blue March, organised in France and Monaco Principality, should therefore be encouraged[35].
The absence of organised screening increases the social burden of cancer, delaying the adoption of treatments of proven efficacy that induce as little disability as possible. The situation is especially clear in economically and technologically advanced countries like Switzerland, where a significantly lower demand for testing has been found among low-income than high-income citizens. Conceivably, the problem is even more severe in countries where early detection testing is not provided by the national health care system and is predominantly out of pocket, compounding the vulnerability of the poorer groups in the population. State funding is a problem in several countries, like Turkey, where dedicated cancer screening centres are found in each province and are theoretically easier to reach. In fact, however, the fact that most people have to pay for their tests, since only the poorest and oldest benefit from state help, prevents access by large swathes of the population. It should be stressed that the activation of organised screening programmes is not a net cost to emerging countries[56], but is actually cost-effective[57,58], also considering that early tumour detection considerably reduces subsequent social and health costs[43].
The situation of these countries contrasts with the one characterising small states like Monaco Principality and San Marino, where the tiny population enables a more effective organisation of health care, including prevention. Monaco Principality deserves high praise for extending screening benefits to foreign workers[35].
Finally, gastroenterologists play an important role in CRC screening. They should not view early detection testing as a practice undermining their expertise, but as a resource that adds to their specialist training[59]. The same applies to clinical pathologists: in some countries, like Russia, their training is predominantly oriented to necroscopy[40]: diagnosing living patients is therefore a challenge for all health care figures. The epidemiologist also has a critical role in local screening co-ordination, patient flow organisation, data management, and data transmission to cancer registries, to improve cancer knowledge and treatment in the various districts. GPs are an essential link in the prevention chain, since they have the task of raising the awareness of those who are at risk and of stimulating those who would benefit from screening to undergo testing, without alarming them. In Turkey, for instance, GPs have the task of directing patients to screening[44]. Patient associations and health care professionals should also work to increase the awareness of institutions and law-makers towards screening and its benefits.
In conclusion, cancer screening should be viewed as a key health care tool, also because investing in screening protects the weakest in the population, decreases the social burden of cancer, and reduces all types of health care costs, including those for radical surgery, long-term hospitalisation, and chemotherapy. Finally, screening for those at risk should be stimulated, offered without charge, and adequately publicised irrespective of the health care system organisation in place.
Although colorectal cancer (CRC) is a tumour for which screening has proven efficacy and cost-effectiveness, in several European countries screening implementation is fraught with difficulties. CRC incidence is quite variable among European countries, and the lower rates found in eastern Europe are higher than the world mean.
The Council of Europe has recommended the priority activation of CRC screening programmes. According to a 2008 European Commission report on the diffusion of CRC screening programmes in the EU, only 12 of the then 22 member states had population-based screening programmes; the others were recommended to provide to their citizens equal access to cancer prevention.
The present paper provides a systematic review of the screening programmes that are active in the non-EU 28 members of the Council of Europe, using data collected from institutional websites and from the literature. Besides reviewing the epidemiological data (incidence, 5-year prevalence, and mortality), it undertakes a critical examination of their quality and provides key information on colorectal cancer and the prevention strategies adopted in each country.
The absence of organised screening increases the social burden of cancer, delaying the adoption of treatments of proven efficacy that induce as little disability as possible. Conceivably, the problem is even more severe in countries where early detection testing is not provided by the national health care service and is predominantly out of pocket, compounding the vulnerability of the poorer groups in the population. Notably, the activation of organised screening programmes in emerging countries would actually be cost-effective, also considering that early tumour detection considerably reduces subsequent social and health costs.
Fecal occult blood (FOB) refers to blood in the feces that is not visibly apparent. A fecal occult blood test (FOBT) checks for hidden (occult) blood in the stool (feces); immunochemical test (iFOBT) is based on human hemoglobin antibodies.
This review paper covers recent topics in CRC screening, and is concisely written. The information given is helpful to promote the further advance in the field.
P- Reviewer: Nishiyama M S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Altobelli E, Lattanzi A. Cervical carcinoma in the European Union: an update on disease burden, screening program state of activation, and coverage as of March 2014. Int J Gynecol Cancer. 2015;25:474-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Altobelli E, Lattanzi A. Breast cancer in European Union: an update of screening programmes as of March 2014 (review). Int J Oncol. 2014;45:1785-1792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Altobelli E, Lattanzi A, Paduano R, Varassi G, di Orio F. Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med. 2014;62:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20481] [Article Influence: 2048.1] [Reference Citation Analysis (19)] |
| 5. | European Council, 2003. L 327/34 council recommendation of 2 December 2003 on cancer screening (2003/878/EC). : Off J Eur Union 2003; . |
| 7. | Pawa N, Arulampalam T, Norton JD. Screening for colorectal cancer: established and emerging modalities. Nat Rev Gastroenterol Hepatol. 2011;8:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Burt RW. Colorectal cancer screening. Curr Opin Gastroenterol. 2010;26:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Gupta AK, Brenner DE, Turgeon DK. Early detection of colon cancer: new tests on the horizon. Mol Diagn Ther. 2008;12:77-85. [PubMed] |
| 10. | Von Karsa L, Segnan N, Patnick J. European Guidelines for Quality Assurance in Colorectal Cancer Screening. : Publications Office of the European Union 2010; . [DOI] [Full Text] |
| 11. | European Commission, 2010. P7_TA(2010) 0152 - Commission Communication on Action Against Cancer: European Partnership. European Parliament Resolution of 6 May 2010 on the Commission Communication on Action Against Cancer: European Partnership 2010; . |
| 12. | Benson VS, Atkin WS, Green J, Nadel MR, Patnick J, Smith RA, Villain P. Toward standardizing and reporting colorectal cancer screening indicators on an international level: The International Colorectal Cancer Screening Network. Int J Cancer. 2012;130:2961-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Brenner H, Hoffmeister M, Haug U. Should colorectal cancer screening start at the same age in European countries? Contributions from descriptive epidemiology. Br J Cancer. 2008;99:532-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 864] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 15. | Swan H, Siddiqui AA, Myers RE. International colorectal cancer screening programs: population contact strategies, testing methods and screening rates. Pract Gastroenterol. 2012;36:20-29. |
| 16. | Zavoral M, Suchanek S, Zavada F, Dusek L, Muzik J, Seifert B, Fric P. Colorectal cancer screening in Europe. World J Gastroenterol. 2009;15:5907-5915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171-177. [PubMed] |
| 19. | Guðlaugsdóttir S. Implementing colorectal cancer screening program in Iceland. In: WEO Barcelona October 2015 proceedings of WEO Colorectal Cancer Screening Meeting. Barcelona, Spain. Available from: https://www.researchgate.net/publication/283569319_. |
| 20. | Cancer registry of Norway. Available from: http://www.kreftregisteret.no/en/Cancer-prevention/Screening-for-colorectal-cancer/. |
| 21. | Hoff G, Grotmol T, Skovlund E, Bretthauer M. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 22. | Giordano L, Bisanti L, Salamina G, Ancelle Park R, Sancho-Garnier H, Espinas J, Berling C, Rennert G, Castagno R, Dotti M. The EUROMED CANCER network: state-of-art of cancer screening programmes in non-EU Mediterranean countries. Eur J Public Health. 2016;26:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Cekodhima G, Alimehmeti I, Cekodhima A, Belishta O. Gastrointestinal polypoid lesions: the Albanian reality. RRJMHS. 2015;4:33-37. |
| 24. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/alb_en.pdf?ua=1. |
| 25. | Buturovic S. Colonoscopy as a method of choice in the diagnosis of colorectal cancer. Acta Inform Med. 2014;22:164-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/bih_en.pdf?ua=1. |
| 27. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/mkd_en.pdf?ua=1. |
| 28. | Panic N, Rösch T, Smolovic B, Radunovic M, Bulajic M, Pavlovic-Markovic A, Krivokapic Z, Djuranovic S, Ille T, Bulajic M. Colorectal cancer screening in a low-incidence area: general invitation versus family risk targeting: a comparative study from Montenegro. Eur J Gastroenterol Hepatol. 2015;27:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/mne_en.pdf?ua=1. |
| 30. | Official Gazette of RS. No. 107/05, 72/09: Article 16, paragraph 2 of the Law on Health Care Law, 88/10, 99.10, 57/11, 119/12 and 45/13 ) and Article 42, paragraph 1 of the Law on Government (“Official Gazette of RS”, No. 55/05, 71/05 - correction, 101/07, 65/08, 16/11, 68/12 - US and 72/12). Available from: http://www.skriningsrbija.rs/files/File/English/REGULATION_ON_THE_NATIONAL_PROGRAM_FOR_EARLY_DETECTION_OF_COLORECTAL_CANCER.pdf. |
| 31. | Cancer registry of Serbia. Available from: http://www.skriningsrbija.rs/eng/colorectal-cancer-screening/statistics/. |
| 32. | Fedewa SA, Cullati S, Bouchardy C, Welle I, Burton-Jeangros C, Manor O, Courvoisier DS, Guessous I. Colorectal Cancer Screening in Switzerland: Cross-Sectional Trends (2007-2012) in Socioeconomic Disparities. PLoS One. 2015;10:e0131205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Marbet UA, Bauerfeind P, Brunner J, Dorta G, Valloton JJ, Delcò F. Colonoscopy is the preferred colorectal cancer screening method in a population-based program. Endoscopy. 2008;40:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Auer R, Selby K, Bulliard JL, Nichita C, Dorta G, Ducros C, Cornuz J. [Shared decision making in the colorectal cancer screening program in the canton of Vaud]. Rev Med Suisse. 2015;11:2209-2215. [PubMed] |
| 35. | The Monaco Health Screening Centre. Available from: http://en.gouv.mc/Policy-Practice/Social-Affairs-and-Health/An-exemplary-Public-Health-system/Monaco-Health-Screening-Centre. |
| 36. | Istituto per la sicurezza sociale di San Marino. Available from: http://www.iss.sm/on-line/home/menudestra/screening-prevenzione.html. |
| 37. | Screening del tumore al colon-retto: San Marino è meglio dell’Italia. Il giornale di San Marino 10/06/2014. Available from: http://giornalesm.com/screening-del-tumore-al-colon-retto-san-marino-e-meglio-dellitalia/. |
| 38. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/and_en.pdf?ua=1. |
| 39. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/mda_en.pdf?ua=1. |
| 40. | Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, Liedke PE, Pramesh CS, Badovinac-Crnjevic T, Sheikine Y. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 41. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/rus_en.pdf?ua=1. |
| 42. | Available from: http://globenewswire.com/news-release/2015/11/09/785009/0/en/Colorectal-screening-project-to-start-in-Russia-with-Biohit-Oyj-s-ColonView-test.html. |
| 43. | Starostina MA, Afanasjeva ZA, Hasanov RSh, Borisov DA, Nagumanov EV, Gordeev MG. Screening for colorectal cancer in Tatarstan Republic. Onco Surgery. 2014;6:40-45. |
| 44. | Tatar M, Tatar F. Colorectal cancer in Turkey: current situation and challenges for the future. Eur J Health Econ. 2010;10 Suppl 1:S99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/tur_en.pdf?ua=1. |
| 46. | Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E. Swaminathan R, Ferlay J, editors. . |
| 47. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/ukr_en.pdf?ua=1. |
| 48. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/arm_en.pdf?ua=1. |
| 49. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/aze_en.pdf?ua=1. |
| 50. | WHO cancer country profile. Available from: http://www.who.int/cancer/country-profiles/geo_en.pdf?ua=1. |
| 51. | Davies P, Jugell L. Capacity assessment and recommendations of cancer screening in Geogia, Tblisi. Available from: http://www.ecca.info/fileadmin/user_upload/Reports/UNFPA_Cancer_Screening_in_Georgia.pdf. |
| 52. | Charter of fundamental rights of the European union. Official Journal of the European Communities 2000/C 364/01. Available from: http://www.europarl.europa.eu/charter/pdf/text_en.pdf. |
| 53. | Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine. ETS No. 164. Available from: http://www.coe.int/it/web/conventions/full-list/-/conventions/treaty/164. |
| 54. | Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 907] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 55. | Moffat J, Bentley A, Ironmonger L, Boughey A, Radford G, Duffy S. The impact of national cancer awareness campaigns for bowel and lung cancer symptoms on sociodemographic inequalities in immediate key symptom awareness and GP attendances. Br J Cancer. 2015;112 Suppl 1:S14-S21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Ginsberg GM, Lauer JA, Zelle S, Baeten S, Baltussen R. Cost effectiveness of strategies to combat breast, cervical, and colorectal cancer in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ. 2012;344:e614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33:88-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 58. | Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 376] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 59. | Stanford SB, Lee S, Masaquel C, Lee RH. Achieving competence in colonoscopy: Milestones and the need for a new endoscopic curriculum in gastroenterology training. World J Gastrointest Endosc. 2015;7:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |