Published online Apr 28, 2016. doi: 10.3748/wjg.v22.i16.4259
Peer-review started: December 4, 2015
First decision: December 31, 2015
Revised: January 27, 2016
Accepted: February 20, 2016
Article in press: February 22, 2016
Published online: April 28, 2016
Processing time: 138 Days and 11.5 Hours
Pancreaticoduodenal artery aneurysms are a rare type of visceral artery aneurysm, whose rupture is associated with high mortality. These aneurysms are of particular interest because local haemodynamic change caused by coeliac artery obstruction plays an important role in their development. However, the pathophysiological mechanism of coeliac artery obstruction is not completely understood. Pressure from the median arcuate ligament is most frequently reported cause. Although it is well-known that stenosis or occlusion of the visceral vessels may be caused by aortic syndrome, reports of pancreaticoduodenal artery aneurysm associated with coeliac artery occlusion due to aortic syndrome are extremely rare. Our case indicates a new aetiology for a pancreaticoduodenal artery aneurysm and demonstrates the rapid deterioration of the patient affected.
Core tip: Approximately 60% of patients with pancreaticoduodenal artery aneurysms presented with rupture have an attending mortality rate of 50%. With the development of the device and techniques, transcatheter arterial embolotherapy has decreased the mortality to as low as 0%. Therefore, early detection and treatment is necessary to improve prognosis of the case.
- Citation: Sakatani A, Doi Y, Kitayama T, Matsuda T, Sasai Y, Nishida N, Sakamoto M, Uenoyama N, Kinoshita K. Pancreaticoduodenal artery aneurysm associated with coeliac artery occlusion from an aortic intramural hematoma. World J Gastroenterol 2016; 22(16): 4259-4263
- URL: https://www.wjgnet.com/1007-9327/full/v22/i16/4259.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i16.4259
Sutton and Lawton first reported a pancreaticoduodenal artery (PDA) aneurysm associated with coeliac axis occlusion in 1973 and since then 93 such cases have been reported[1]. Obstruction of the coeliac artery leads to collateralization of the hepatic and splenic arteries through the superior mesenteric artery (SMA). Increased blood flow in the small and fragile pancreaticoduodenal arcade vessels may contribute to aneurysm development[1,2]. Here we report what is, to the best of our knowledge, the second case in the English-language literature of a PDA aneurysm associated with occlusion of the coeliac artery due to aortic syndrome.
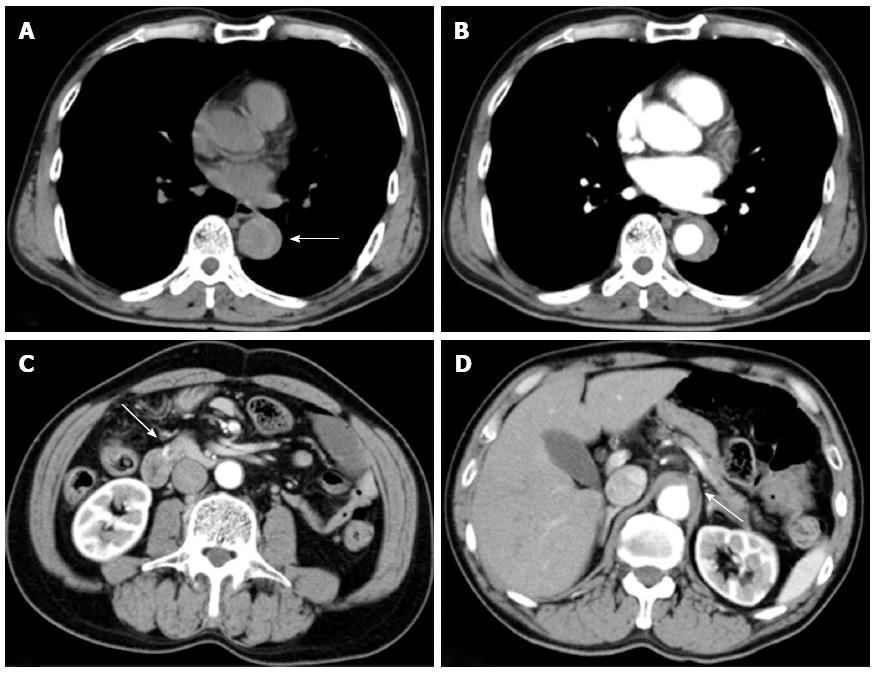
An apparently healthy 60-year-old man developed sudden and severe upper abdominal pain and was admitted to a local hospital. He did not feel nausea or other symptoms of visceral ischemia. There was no history of any cardiovascular disorder, connective tissue disorders, chest injury, and familial history of aortic syndrome. The aortic dissections detection risk score was 1. Initial plain computed tomography (CT) performed 5 h after onset of symptoms showed a dilated descending aorta and hyperattenuated collection located eccentrically within the aortic wall (Figure 1A). However, the attending physician could not recognize it and the cause of abdominal pain remained unclear. Therefore, contrast-enhanced CT had to be performed 6 h after the onset of symptoms, which revealed crescentic and asymmetric wall thickening of the descending aorta: Contrast is not visualized within the aortic media. Intimal flap was not detected. A diagnosis of aortic intramural hematoma (IMH) extending from the distal aortic arch to the level of the coeliac artery was made (Figure 1B-D). The root of the coeliac artery was compressed by IMH (Figures 1D and 2A). The patient was transferred to our hospital 8 h after symptom onset. This patient was not administered any drug which would affect the coagulation during the period of pre-hospital to hospital care.
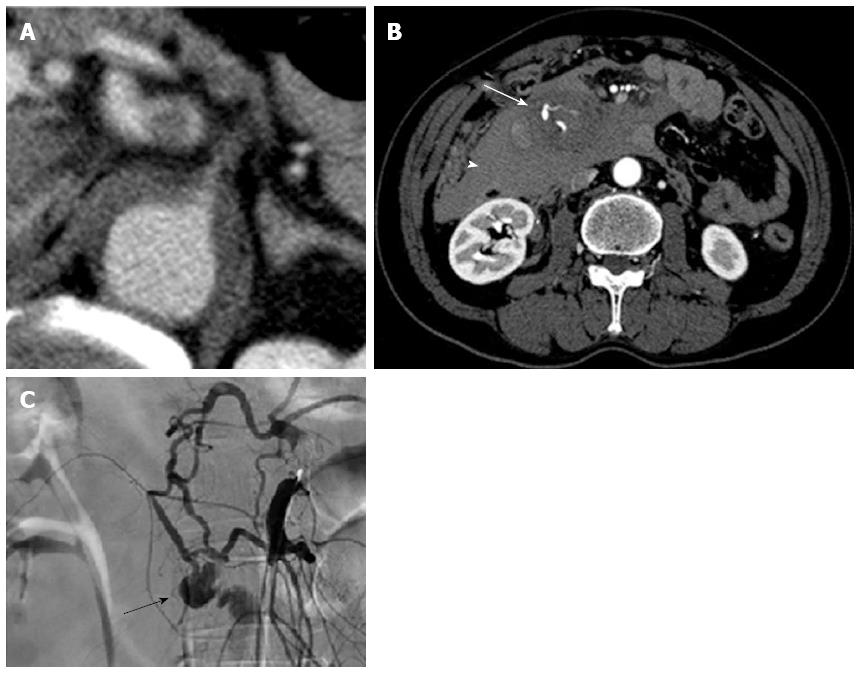
On arrival, his vital signs were normal and initial laboratory data were: white blood cell count 20190/mm3, D-dimer 85.9 μg/mL, and C-reactive protein 3.0 mg/dL, with other parameters, including haemoglobin, prothrombin time, activated partial thromboplastin time, and serum amylase levels within normal limits. However, the patient lost consciousness and became hypotensive (74/40 mmHg) during transfer to the CT room 10 min after arrival in the emergency room. Contrast-enhanced CT revealed an aortic IMH and a large retroperitoneal haematoma involving the head of the pancreas and duodenum (Figure 2A and B).
The patient underwent visceral angiography for localization of the site of injury and to control bleeding. The arteriogram showed complete occlusion at the root of the coeliac artery, collateralization from SMA through the pancreaticoduodenal arcade and a saccular aneurysm of the inferior PDA (Figure 2C). The patient was, therefore, diagnosed with retroperitoneal bleeding caused by a ruptured PDA aneurysm. Selective microcoil embolization proximal and distal to the aneurysm was successfully performed. A clear CT performed at our hospital revealed anterior branch aneurism of inferior PDA (Figure 2B). The patient recovered completely and has been doing well for 5 mo.
PDA aneurysms are rare and account for 2% of all the splanchnic artery aneurysms[3]. PDA aneurysms may either be true aneurysms, resulting from a primary abnormality of the vessel wall, or false aneurysms, resulting from injury and erosion of the vessel wall, most commonly after trauma or inflammation (e.g., pancreatitis). Most cases of true PDA aneurysm are reported in patients in their 50s[1,2], suggesting a different aetiology from that of atherosclerotic aneurysms reported in the elderly. In fact, coeliac axis stenosis has been identified in 60%-75% of patients diagnosed with true PDA aneurysm[4]. In contrast, the reported incidence of coeliac axis stenosis ranges from 12.5% to 24% in the general population[2].
Obstruction of the coeliac artery leads to collateralization of the hepatic and splenic arteries through the SMA. Increased blood flow in the small and fragile pancreaticoduodenal arcade vessels is likely to contribute to aneurysm development[1]. However, the pathophysiological mechanism of coeliac artery obstruction remains to be elucidated. Pressure from the median arcuate ligament (MAL) is the most frequently reported cause[1]. Although it is well known that spontaneous aortic syndrome may cause occlusion of the aortic side branch, to the best of our knowledge, only one Japanese patient with ruptured PDA aneurysm associated with acute aortic syndrome involving the coeliac axis has been reported[5]. In that report, CT at symptom onset confirmed the presence of MAL with acute aortic dissection. Therefore, the authors speculated that both MAL and aortic dissection played a role of causative factor to develop the aneurysm. However, our patient showed no other factor causing coeliac artery occlusion without aortic IMH. Aortic IMH are considered as a variant of aortic dissection, accounting for 10%-30% of all acute aortic syndromes. Despite the difference in the pathophysiology, the prognosis for thoracic aortic dissections and IMH is similar. Although IMH is less likely to be associated with the branch artery occlusion compared with the typical aortic dissection, there have been some reports on ischemia due to IMH[6-8].
Although the duration of the coeliac artery occlusion from aortic IMH was limited in our case, we speculate that this is a causative factor contributing to PDA aneurysm development and rupture because of an interesting characteristic of PDA aneurysms. Although most visceral artery aneurysms show a correlation between the size and the frequency of rupture, this does not apply to PDA aneurysms[1,9,10]. Because true aneurysms of the splenic and hepatic artery, accounting for 70% of all visceral artery, rarely rupture when they are < 2 cm[11,12], it is generally believed that visceral aneurysm require a lot of time to develop and rupture. However, PDA aneurysms have not shown a clear correlation between size and propensity to rupture[1,9,10]. Brocker et al[1] reported a size variance from 3 to 40 mm (mean 15.2 mm) for ruptured PDA aneurysm. In his report, at least 24 patients bled from aneurysm < 2 cm and 14 patients bled from aneurysm < 1 cm. This suggests PDA aneurysms may rupture within short time after development. Therefore, we conclude that the celiac artery obstruction due to aortic IMH played an important role in the development and rupture of PDA aneurysm in this case, even in a limited duration of time.
It is difficult to make diagnosis of PDA aneurysms before the occurrence of rupture because of the low prevalence and nonspecific symptoms. Approximately 60% of patients with PDA aneurysms presented with rupture have an attending mortality rate of 50%[2]. With the development of the device and techniques, transcatheter arterial embolotherapy has decreased the mortality to as low as 0%[2]. Therefore, early detection and treatment is necessary to improve prognosis of the case.
An apparently healthy 60-year-old man diagnosed aortic intramural hematoma at local hospital lost consciousness and became hypotensive after transfering to Otemae Hospital.
Type B aortic intramural hematoma complicated retroperitoneal bleeding of a ruptured pancreaticoduodenal artery (PDA) aneurysm.
Acute aortic ruputure, cardiac tamponade.
Initial laboratory data showed elevation only of white blood cell count, D-dimer and C-reactive protein.
Contrast-enhanced computed tomography showed retroperitoneal hematoma and pancreaticoduodenal artery aneurysm associated with occlusion of the coeliac artery due to aortic intramural hematoma.
Transcatheter arterial embolization.
Only one Japanese patient with ruptured PDA aneurysm associated with acute aortic syndrome involving the coeliac axis has been reported.
Aortic intramural hematoma is a life-threatening aortic disease included within acute aortic syndrome, together with aortic dissection and penetrating aortic ulcer.
PDA aneurysms, which is uncommon but life-threatening complications may occur in patients with aortic syndrome involving the visceral arteries.
This case report describes the case of a 60-year old man affected by type B aortic intramural hematoma, complicated by retroperitoneal bleeding of a ruptured PDA aneurysm.
P- Reviewer: Gao BL, Morello F S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Brocker JA, Maher JL, Smith RW. True pancreaticoduodenal aneurysms with celiac stenosis or occlusion. Am J Surg. 2012;204:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Dave B, Sharma A, Kwolek C, Demoya M, Wicky S, Kalva S. Percutaneous transcatheter arterial embolization of inferior pancreatico-duodenal artery aneurysms associated with celiac artery stenosis or occlusion. Catheter Cardiovasc Interv. 2010;75:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Lakin RO, Kashyap VS. Splanchnic Artery Aneurysms. Rutherford’s Vascular Surgery. 8th ed. Philadelphia: W.B.Saunders 2014; 2220-2235. |
| 4. | Roger DB. Personal space, body image and leadership: an exploratory study. Percept Mot Skills. 1976;43:25-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Miyayama S, Terada T, Tamaki M. Ruptured pancreaticoduodenal artery aneurysm associated with median arcuate ligament compression and aortic dissection successfully treated with embolotherapy. Ann Vasc Dis. 2015;8:40-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Sawhney NS, DeMaria AN, Blanchard DG. Aortic intramural hematoma: an increasingly recognized and potentially fatal entity. Chest. 2001;120:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Evangelista A, Mukherjee D, Mehta RH, O’Gara PT, Fattori R, Cooper JV, Smith DE, Oh JK, Hutchison S, Sechtem U. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | von Kodolitsch Y, Csösz SK, Koschyk DH, Schalwat I, Loose R, Karck M, Dieckmann C, Fattori R, Haverich A, Berger J. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation. 2003;107:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Ducasse E, Roy F, Chevalier J, Massouille D, Smith M, Speziale F, Fiorani P, Puppinck P. Aneurysm of the pancreaticoduodenal arteries with a celiac trunk lesion: current management. J Vasc Surg. 2004;39:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Suzuki K, Kashimura H, Sato M, Hassan M, Yokota H, Nakahara A, Muto H, Yuzawa K, Fukao K, Tanaka N. Pancreaticoduodenal artery aneurysms associated with celiac axis stenosis due to compression by median arcuate ligament and celiac plexus. J Gastroenterol. 1998;33:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Lakin RO, Bena JF, Sarac TP, Shah S, Krajewski LP, Srivastava SD, Clair DG, Kashyap VS. The contemporary management of splenic artery aneurysms. J Vasc Surg. 2011;53:958-64; discussion 965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Abbas MA, Stone WM, Fowl RJ, Gloviczki P, Oldenburg WA, Pairolero PC, Hallett JW, Bower TC, Panneton JM, Cherry KJ. Splenic artery aneurysms: two decades experience at Mayo clinic. Ann Vasc Surg. 2002;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |