Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2550
Peer-review started: July 8, 2014
First decision: August 6, 2014
Revised: August 28, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: February 28, 2015
Processing time: 235 Days and 20 Hours
Hepatocyte nuclear factor 1-β (HNF1B) defects cause renal cysts and diabetes syndrome (RCAD), or HNF1B-maturity-onset diabetes of the young. However, the hepatic phenotype of HNF1B variants is not well studied. We present a female neonate born small for her gestational age [birth weight 2360 g; -2.02 standard deviations (SD) and birth length 45 cm; -2.40 SD at the 38th gestational week]. She developed neonatal cholestasis due to biliary atresia and required surgical intervention (portoenterostomy) when 32-d old. Following the operation, icterus resolved, but laboratory signs of liver dysfunction persisted. She had hyperechogenic kidneys prenatally with bilateral renal cysts and pancreatic hypoplasia postnatally that led to the diagnosis of an HNF1B deletion. This represents the most severe hepatic phenotype of an HNF1B variant recognized thus far. A review of 12 published cases with hepatic phenotypes of HNF1B defects allowed us to distinguish three severity levels, ranging from neonatal cholestasis through adult-onset cholestasis to non-cholestatic liver impairment, all of these are associated with congenital renal cysts and mostly with diabetes later in life. We conclude that to detect HNF1B variants, neonates with cholestasis should be checked for the presence of renal cysts, with special focus on those who are born small for their gestational age. Additionally, patients with diabetes and renal cysts at any age who develop cholestasis and/or exocrine pancreatic insufficiency should be tested for HNF1B variants as the true etiological factor of all disease components. Further observations are needed to confirm the potential reversibility of cholestasis in infancy in HNF1B mutation/deletion carriers.
Core tip: Hepatocyte nuclear factor 1-β (HNF1B) defects cause renal cysts and diabetes syndrome (renal cysts and diabetes; HNF1B-maturity-onset diabetes of the young), but little is known on liver in these patients. We succeeded to detect the most severe hepatic phenotype of an HNF1B gene deletion in a female neonate with cholestasis due to biliary atresia. She required portoenterostomy when 32-d old. She had bilateral renal cysts and pancreatic hypoplasia. A review of 12 published cases allows distinguishing three severity levels of liver impairment in HNF1B defects, ranging from neonatal cholestasis through adult-onset cholestasis to non-cholestatic liver disease. All have renal cysts and later-onset diabetes.
-
Citation: Kotalova R, Dusatkova P, Cinek O, Dusatkova L, Dedic T, Seeman T, Lebl J, Pruhova S. Hepatic phenotypes of
HNF1B gene mutations: A case of neonatal cholestasis requiring portoenterostomy and literature review. World J Gastroenterol 2015; 21(8): 2550-2557 - URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2550.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2550
The hepatocyte nuclear factors (HNFs) are key transcriptional regulators of embryonic and fetal liver development and differentiation of the biliary system. Their life-long expression in human hepatocytes (HNF4A and HNF6) and in biliary epithelial cells (HNF1B, HNF3A and HNF3B) suggests that they have a role in postnatal cell survival, function and regeneration[1-3]. Of these, the hepatocyte nuclear factor 1-β (HNF1B), encoded by the HNF1B gene, is involved in transcriptional and functional regulation not only of the liver and biliary system but also of the kidneys, urogenital tract and pancreatic β-cells. Deficiency of this gene due to HNF1B point mutations or whole gene deletions was first recognized in a small subgroup of patients with maturity-onset diabetes of the young (MODY) and was originally designated as MODY5[4-6]. It has been demonstrated that HNF1B is involved in regulating the β-cell transcription factor network[7]. Due to the frequent co-occurrence of MODY5 diabetes with renal cysts and/or other inborn urogenital abnormalities, the syndrome associated with HNF1B defects is now referred to as RCAD (renal cysts and diabetes). In a recent single centre study, 9% of adult patients with chronic renal failure carried a pathogenic HNF1B variant[8]. Most of the HNF1B variants are de novo whole gene deletions[9-12]. However, the hepatic and biliary phenotype of HNF1B defects has been recognized only in single patients up to this point.
In mice, HNF1B is expressed in the embryonic gall bladder, liver and intrahepatic bile ducts[13]. Furthermore, it is expressed in the adult liver. It has been shown to co-regulate morphogenesis of the biliary system[14]. HNF1B knockout mice suffer from severe neonatal cholestasis and jaundice due to the abnormally developed gall bladder and dysfunctional intrahepatic bile ducts. In addition, HNF1B and HNF1A control genes that affect bile acid transport and metabolism[14,15].
Kitanaka et al[16] first described a patient with neonatal cholestasis, liver dysfunction and hypercholesterolemia caused by a heterozygous mutation, H153N, in HNF1B. The patient later developed non-autoimmune diabetes and was diagnosed with bilateral renal cysts. Two of his paternal ancestors died from hepatic cancer and liver cirrhosis, and multiple family members suffered from chronic renal insufficiency and/or liver disease. Unfortunately, a detailed phenotypic characterization and genotyping of his family members was unavailable.
Additionally, two patients with severe hepatic and biliary phenotype due to monoallelic HNF1B mutations/deletions were described by Beckers et al[17] and by Raile et al[18]. Both patients presented with severe neonatal jaundice, a paucity of intrahepatic bile ducts at liver biopsy and a tendency toward improvement in cholestasis in the late stages of the first year of life. Recently, another patient with a de novo HNF1B mutation, S148L, and renal and hepatic dysfunction diagnosed at 3 mo of age was reported in Turkey when he was investigated for failure to thrive[19]. However, he had no signs of cholestasis.
Adult-onset cholestasis due to HNF1B mutations developed in three patients with known diabetes; the first signs of cholestasis were noted at ages 33, 53 and 30 years[20]. The authors found normal anatomy of the intra- and extrahepatic bile ducts, but the patients were lacking primary cilia on their cholangiocytes. The authors proposed that HNF1B mutations might be classified as ciliopathy.
The female patient reported here is the offspring of healthy unrelated parents of Czech origin. There was no family history of known diabetes, hepatic or renal disease. She was born from a first pregnancy in the 38th gestational week with a birth weight of 2360 g [-2.02 standard deviation (SD)] and birth length of 45 cm (-2.40 SD; according to normative data[21]). Fetal hypotrophy and hyperechogenic kidneys were recognized by ultrasound at the 30th gestational week.
Since the first day of life, she had apparently acholic stools and gradually developed jaundice. At day 4, her total bilirubin was 104 μmol/L and her conjugated bilirubin was 32 μmol/L. Her level of γ-glutamyltransferase was markedly increased (23.3 μkat/L), but her levels of alkaline phosphatase (2.3 μkat/L) and alanine-aminotransferase (0.42 μkat/L) were normal. Her aspartate-aminotransferase level was mildly elevated (1.27 μkat/L) (Table 1).
| Age | Total bilirubin,μmol/L | Conjugated bilirubin,μmol/L | AST,μkat/L | ALT,μkat/L | GGT,μkat/L | ALP,μkat/L | Urea, mmol/L | Creatinine,μmol/L | Cholesterol, mmol/L | Albumin, g/L | INR | Mg, mmol/L |
| 4 d | 104 | 32 | 1.27 | 0.42 | 23.3 | 2.3 | 4.7 | 81 | 2.7 | 36.0 | 0.91 | NA |
| 27 d | 145 | 100 | 1.57 | 0.92 | 10.2 | 7.2 | 2.2 | 29 | 3.7 | 36.8 | 1.05 | NA |
| 5 mo | 135 | 110 | 2.59 | 2.24 | 6.8 | 8.1 | 5.2 | 22 | 9.5 | 42.0 | 0.84 | NA |
| 19 mo | 30 | 25 | 3.15 | 3.09 | 7.5 | 11.4 | 5.3 | 18 | 6.7 | 42.7 | 0.95 | 0.90 |
| 2 yr | 33 | 28 | 3.10 | 3.13 | 6.4 | 10.7 | 5.5 | 21 | 6.7 | 43.9 | 0.92 | 0.66 |
Abdominal ultrasound revealed bilateral renal cysts with diameters up to 5 mm. Her liver had normal echogenicity and echotexture; however, her gallbladder was small. Her pancreas was hypoplastic with absent body and tail, the spleen was normal, and no additional abnormalities were found. Her kidney function was normal. An infectious, metabolic or immunological basis for neonatal cholestasis was not found.
Due to progressive conjugated hyperbilirubinemia and acholic stools, endoscopic retrograde cholangiopancreatography (ERCP) was performed at 30 d of age. Normal pancreatic ducts were observed, but extrahepatic bile ducts were unrecognizable, which led to the indication for explorative surgery that was then provided at 32 d. Surgery revealed an atrophic gallbladder. Her choledochus and proximal extrahepatic bile ducts were completely atretic and had been replaced by connective tissue. The surgeon performed portoenterostomy sec. Kasai. Liver histology from a preoperatively obtained wedge biopsy showed cholestasis without signs of gigantocellular hepatocyte transformation or bile duct proliferation. Portal fields were dilated with connective tissue, having thin or atretic bile ducts. Close to the porta hepatis, the configuration of bile ducts resembled the findings typical of biliary atresia.
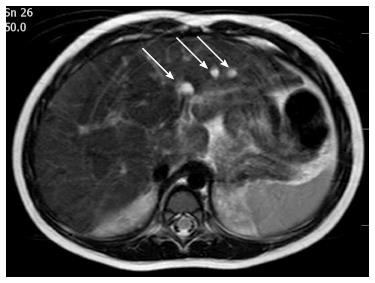
Following surgery, the color of her stools normalized, and both the bilirubin and γ-glutamyltransferase levels declined to mildly elevated values. Post-operative ultrasound follow-up revealed abnormal echotexture of her left hepatic lobe that was suspicious of a cystic malformation. A magnetic resonance cholangiopancreatography (MRCP) identified multiple cystic dysplasia of the left hepatic lobe at 2 years of age. Seven cysts were found with diameters ranging from 2 to 7 mm (Figure 1). By the current age of 2 years, she is growing along the 3rd centile and her PELD score is “1”. She has normal blood glucose, fecal elastase activity and renal function. Mild hypomagnesemia was observed (0.66 mmol/L).
Following the parents’ written consent, the child was included into a study to investigate genetic causes of primary biliary atresia. The study was previously approved by the institutional ethical committee of the University Hospital Prague-Motol. Genetic testing was initiated by direct sequencing of the JAG1 gene, mutations in which are recognized as a major cause of Alagille syndrome and some additional cases of severe biliary atresia[22]. The findings were negative. The investigations continued with testing for HNF1B using Multiple Ligation Probe-dependent Amplification (MLPA) and subsequent array Comparative Genomic Hybridization methods. She was found to carry an entire HNF1B gene deletion spanning 1698 kb, which was previously reported as causative in a patient with MODY diabetes[23]. None of her parents carry an HNF1B gene deletion, indicating that the mutation has arisen de novo in the proband.
Most of the published cases with HNF1B variants were assessed for the presence of diabetes and/or renal impairment, mostly renal cysts. To the best of our knowledge, five detailed published reports have focused on the hepatic phenotype in HNF1B mutation/whole gene deletion carriers. From these reports, and with the addition of our own observations, we managed to collect clinical and laboratory data on liver disease in 12 subjects, which are summarized in Table 2.
| Ref. | Sex/Origin | GW/BW g (BW SDS) (Lawrence 1989) | First clinical symptoms (age) | Laboratory results | Kidney ultrasound | Kidney function | Liver biopsy | Pancreas morphology | Pancreatic exocrine function | Onset of clinical diabetes (age) | Diabetes treatment | Intellectual development | Genotype (inherited from) |
| Severe neonatal cholestatic icterus | |||||||||||||
| Present report | F/Czech | 38/2360 (-2.02) | Neonatal cholestasis (first week of life) | Anemia; progressively increasing, mainly cholestatic, liver tests | Multiple bilateral cortical cysts (maximal diameter 5 mm), prenatally hyperechogenic kidneys | Normal by 2 yr | Paucity of intrahepatic bile ducts, severe biliary stasis, marked periportal fibrosis | Absent body and tail | Normal fecal elastase by age 19 mo | Normoglycaemia by 2 yr | Not applicable | Normal | 1698 kb deletion including HNF1B (de novo) |
| [17] | M/Sardinian | 37/1520 (-3.70) | Neonatal cholestasis (first weeks of life) | High but resolving hyperbilirubinemia; fluctuating liver enzymes; high triglyceridemia | Left kidney agenesis; enlarged and hyperechogenic right kidney; multiple cortical cysts | Progressive chronic renal insufficiency by 18 yr | Paucity of intrahepatic bile ducts, severe biliary stasis, slight periportal fibrosis | Progressive atrophy from birth up to 16 yr | Progressive decline; need for enzyme replacement by 16 yr | Transient neonatal hyperglycemia; permanent diabetes since 5 yr | Insulin 1.26 U/kg per day by 18 yr | NA | c. 499_504delGCTC TGinsCCCCT (de novo) |
| [16] | M/Japanese | 39/2390 (-2.54) | Neonatal respiratory distress; neonatal cholestasis (first weeks of life) | Hyperbilirubinemia (resolving by 9 mo); transiently high cholesterol; constantly high AST, ALT | Multiple bilateral cysts (right, four cysts of 10-20 mm diameter; left, one cyst of 10 mm diameter) | Mild chronic renal insufficiency by 13 yr | Marked cholestasis; reduced number of intrahepatic bile ducts; no signs of infiltration | NA | NA | 13 yr | Insulin 0.4 U/kg per day after therapy onset | Slightly delayed | c. 457C>A (de novo or paternal) |
| Late-onset cholestasis | |||||||||||||
| [18] case No. 1 | M/German | 35/1780 (-2.08) | Neonatal cholestasis (first weeks of life) | Elevated AST/ALT/GGT | Cystic dysplasia; hydronephrosis due to urethral stenosis | Chronic renal insufficiency by 18 yr | Intrahepatic cholestasis due to paucity of bile ducts | Hypoplastic | Fecal elastase deficiency | 13 yr | Insulin 1.34 U/kg per day by 18 yr | Retarded | 1590 kb deletion including HNF1B (de novo or paternal) |
| [20] case No. 1 | F/? | NA | Jaundice (29 yr) | Progressively increasing, mainly cholestatic, liver tests; hypomagnesaemia | Renal cysts | Mild chronic renal insufficiency by 33 yr | Non-specific changes; slight steatosis | Atrophic | NA | 14 yr | NA | Normal | 1423 kb deletion including HNF1B (NA) |
| [20] case No. 2 | M/? | NA | "Chronic pancreatitis" (44 yr) | Progressively increasing, mainly cholestatic, liver tests; hypomagnesaemia | Renal cysts | Mild chronic renal insufficiency by 53 yr | Minor sinusoidal dilatation | Atrophic | NA | 51 yr | NA | Retarded | 1427 kb deletion including HNF1B (NA) |
| Non-cholestatic liver impairment | |||||||||||||
| [20] case No. 3 | F/? | NA | Jaundice (30 yr) | Progressively increasing cholestatic liver tests; hypomagnesaemia | Renal cysts | Chronc renal insufficiency by 30 yr | Thickened basal membranes around the bile ducts; minor sinusoidal dilatation | Atrophic | NA | Before 30 yr | NA | Normal | c. 544C>T (NA) |
| [18] case No. 2 | F/Turkish | 40/2630 (-2.13) | Unilateral cystic kidney dysplasia; diabetes (13 yr) | Elevated AST/ALT | Unilateral cystic dysplasia | Normal by 15 yr | No cholestasis | Normal | Normal | 13 yr | Insulin 0.24 U/kg per day by 15 yr | Normal | 1700 kb deletion including HNF1B (de novo) |
| [18] case No. 3 | F/Vietnamese | 40/2600 (-2.20) | Prolonged severe hyperglycemia; bilateral cataracts (16 yr) | Elevated AST/ALT/GGT | Bilateral cortical cysts | Mild chronic renal insufficiency by 16.5 yr | No cholestasis | Absent body and tail | Fecal elastase deficiency | 16 yr | Insulin 3.4 U/kg per day by 16.5 yr | Normal | 1310 kb deletion including HNF1B (de novo) |
| [18] case No. 4 and [28] | M/German | 38/2650 (-1.53) | Failure to thrive and renal failure (4 wk) | Elevated AST/ALT/GGT | Hypoplastic hyperechogenic kidneys; cortical cysts | End-stage renal failure - dialysis since 10 yr, transplantation at 10.5 yr | Steatohepatitis | Normal | Normal | 15 yr | Insulin 0.2 U/kg per day by 15 yr | Retarded | 1550 kb deletion including HNF1B (de novo) |
| [18] case No. 5 | F/German | 40/3340 (-0.40) | Unilateral coloboma and loss of vision; diabetes (12 yr) | Elevated AST/ALT | No visible renal abnormity; arterial hypertension | Normal by 15.6 yr | Steatohepatitis | Normal | Normal | 12 yr | Insulin 0.4 U/kg per day by 15.6 yr | Normal | 1430 kb deletion including HNF1B (de novo or paternal) |
| [19] | F/Turkish | 38/1900 (-3.13) | Fever; diarrhea; failure to thrive (3 mo) | Anemia; leucocytosis; renal and hepatic dysfunction; acidosis; hyperglycemia | Hypoplastic hyperechogenic kidneys; cortical cysts | Progressive chronic renal insufficiency by 7 yr | Mild steatosis; iron deposition in hepatocytes and Kupffer cells; persistent hematopoiesis | Atrophic head; absent body and tail | Fecal elastase deficiency | Transient hyperglycemia at 3 mo; permanent diabetes since 6 yr | Insulin 0.42 U/kg per day after therapy onset | NA | c. 443C>T (de novo) |
Interestingly, the detailed hepatic phenotypes available for the 12 patients with pathogenic HNF1B variants tend to cluster into three severity degrees of liver dysfunction, ranging from neonatal cholestasis through late-onset cholestasis to the mildest form and non-cholestatic liver impairment.
Four patients who manifested with progressive neonatal cholestasis within first weeks of life have shown the most severe phenotypes. All of them were born small for their gestational age, with birth weights below -2 SD after adjustment for gestational age. Because only one of them manifested transient neonatal hyperglycemia, the intrauterine growth restriction was very unlikely to be due to insulin deficiency. The reason for this growth restriction is still unknown[15-17].
All four patients had similar findings at liver biopsy, including marked cholestasis, a paucity of intrahepatic bile ducts and a variable degree of periportal fibrosis. Our patient fulfilled the strict clinical criteria for surgical intervention due to severe biliary atresia with cholestasis and underwent portoenterostomy at 32 d of age, but three additional patients with apparently milder cholestasis only underwent conservative therapy. In at least two of them, hyperbilirubinemia partially resolved by the first birthday. All of them have multiple renal cysts (with additional unilateral kidney agenesis and a hyperechogenic contralateral kidney with multiple cortical cysts in one case), and those who already reached in their second decade of life began proceeding to chronic renal insufficiency. Typically, they have pancreatic hypoplasia (with documented progressive pancreatic atrophy in one case) and impaired pancreatic exocrine function. Overt diabetes typically manifests within the first two decades of life and requires insulin therapy. Their intellectual development is variable.
The intermediate hepatic phenotype - late-onset cholestasis - was reported in three patients[20]. Their cholestasis first manifested at 29-44 years of age with accordingly milder bioptic findings, presumably minor sinusoidal dilatation. All of them had concomitant renal involvement with renal cysts. However, their chronic renal insufficiency tended to occur later than in the former subgroup and was recognized when they were middle-aged adults. Their pancreas is atrophic, but data on their exocrine function are not available. Diabetes manifested between 14 and 51 years of life. Similar to the previous group, the intellectual development is variable.
The mildest hepatic phenotype relative to the other phenotypes includes five patients with non-cholestatic liver impairment[18,19]. They had a tendency toward intrauterine growth restriction and a variable first clinical presentation, ranging from failure to thrive or renal failure in infancy up to diabetic symptoms in the second decade of life. Renal cysts were present in most of these patients, but chronic renal insufficiency developed only in three of the five by the second decade of life. Their liver enzymes were clearly elevated; however, histological findings from the liver biopsies were milder than those from the previous subgroups. Diabetes manifested in all subjects within their first or second decade of life and required insulin administration in all cases. This less severe hepatic phenotype was also more frequently linked with normal pancreas morphology, normal exocrine function and, with one exception, normal intellectual development.
A similar phenotype was described in a family with four affected subjects[11].
Regarding genetic findings, there was no apparent phenotypic difference between subjects with single base mutations or whole gene deletions.
Our analysis of the available clinical observations of the hepatic phenotype in HNF1B mutation/deletion carriers raises the following clinical implications for neonates, children and adults with cholestasis: (1) neonates with severe cholestasis should undergo renal ultrasound focusing on renal cortical cysts and additional renal developmental abnormalities. A concurrence of neonatal cholestasis with renal cysts is suspicious for an HNF1B defect; (2) in patients with neonatal cholestasis, a history of an intrauterine growth restriction is supportive of an HNF1B defect because the majority of other children with neonatal cholestasis have normal birth weight and birth length (unpublished observations of 96 neonates with biliary atresia). Interestingly, in HNF1B mutation/deletion carriers without a hepatic and/or biliary phenotype, IUGR is rare[24,25], suggesting that the co-occurrence of abnormal HNF1B and biliary atresia predisposes individuals to diminished intrauterine growth and weight gain; (3) as demonstrated by other cases in which neonatal cholestasis due to an HNF1B defect spontaneously resolved within the first year of life, further clinical evidence is needed to develop recommendations on the optimal management for affected children; (4) patients of any age with known diabetes who develop pancreatic exocrine insufficiency and/or cholestasis should be investigated for renal cysts; and (5) if positive, they are highly likely to have an HNF1B defect. In these patients, the etiological genetic diagnosis may clarify the origin of the hepatic and/or exocrine pancreatic disease, which is not a complication of diabetes but rather an additional component of the primary disease. Hypomagnesaemia may be a strong supportive finding in favor of an HNF1B testing as it was demonstrated to occur in about half of subjects with HNF1B defects[26]. We propose to incorporate these clinical observations into an update of the general selection criteria for HNF1B gene analysis[27].
In conclusion, the hepatic phenotype in HNF1B mutation/deletion carriers clusters into three different levels of severity, ranging from severe neonatal cholestasis through late-onset cholestasis in middle-aged adults up to a mild hepatic phenotype on the background of other, more pronounced symptoms and signs, such as diabetes and renal disease. However, a correct etiological diagnosis is undoubtedly beneficial for all three subgroups of patients, allowing not only a clear understanding of the underlying cause but also a prediction of the risks of additional disease components, including diabetes and exocrine pancreatic dysfunction, which are highly likely to develop within the subsequent years or decades of life.
A female neonate who was born small for her gestational age had apparently acholic stools since the first day of life, and gradually developed jaundice.
The clinical diagnosis was suggestive of neonatal cholestasis due to biliary atresia.
Most children with neonatal cholestasis have isolated biliary atresia; however, some of them may have a syndromic condition.
Laboratory findings at day 4 confirmed cholestatic jaundice with elevated levels of total (104 μmol/L) and conjugated bilirubin (32 μmol/L) and markedly increased level of gamma-glutamyltransferase (23.3 μkat/L).
Abdominal ultrasound revealed bilateral renal cysts, liver with normal echogenicity and echotexture, a small gallbladder and hypoplastic pancreas; endoscopic retrograde cholangiopancreatography showed normal pancreatic ducts, but extrahepatic bile ducts were unrecognizable.
Molecular genetic testing using Multiple Ligation Probe-dependent Amplification (MLPA) lead to recognition of a de novo HNF1B gene deletion spanning 1698 kb, which was previously reported as causative in a patient with MODY diabetes.
Explorative surgery provided at age 32 d revealed an atrophic gallbladder and completely atretic choledochus and proximal extrahepatic bile ducts; the surgeon performed portoenterostomy sec. Kasai.
Most of the published cases with HNF1B variants were assessed for the presence of diabetes and/or renal impairment, mostly renal cysts; only five reports have focused on the hepatic phenotype which was always milder than that observed in the patient.
MLPA is a method of genetic testing directed towards recognition of whole gene deletions.
This observation represents the most severe hepatic phenotype of an HNF1B variant recognized thus far.
The manuscript is really attractive and worthy, and the content is prospective, which is relevant and well presented for publication.
P- Reviewer: Kaymakoglu S, Kuo SM, Zhang YP S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Limaye PB, Alarcón G, Walls AL, Nalesnik MA, Michalopoulos GK, Demetris AJ, Ochoa ER. Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Lab Invest. 2008;88:865-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Raynaud P, Carpentier R, Antoniou A, Lemaigre FP. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2011;43:245-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol. 2012;56:1159-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997;17:384-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 622] [Cited by in F6Publishing: 566] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 5. | Nishigori H, Yamada S, Kohama T, Tomura H, Sho K, Horikawa Y, Bell GI, Takeuchi T, Takeda J. Frameshift mutation, A263fsinsGG, in the hepatocyte nuclear factor-1beta gene associated with diabetes and renal dysfunction. Diabetes. 1998;47:1354-1355. [PubMed] [Cited in This Article: ] |
| 6. | Beards F, Frayling T, Bulman M, Horikawa Y, Allen L, Appleton M, Bell GI, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor 1beta are not a common cause of maturity-onset diabetes of the young in the U.K. Diabetes. 1998;47:1152-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Wang L, Coffinier C, Thomas MK, Gresh L, Eddu G, Manor T, Levitsky LL, Yaniv M, Rhoads DB. Selective deletion of the Hnf1beta (MODY5) gene in beta-cells leads to altered gene expression and defective insulin release. Endocrinology. 2004;145:3941-3949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Musetti C, Quaglia M, Mellone S, Pagani A, Fusco I, Monzani A, Giordano M, Stratta P. Chronic renal failure of unknown origin is caused by HNF1B mutations in 9% of adult patients: a single centre cohort analysis. Nephrology (Carlton). 2014;19:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Lindner TH, Njolstad PR, Horikawa Y, Bostad L, Bell GI, Sovik O. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum Mol Genet. 1999;8:2001-2008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 232] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Kolatsi-Joannou M, Bingham C, Ellard S, Bulman MP, Allen LI, Hattersley AT, Woolf AS. Hepatocyte nuclear factor-1beta: a new kindred with renal cysts and diabetes and gene expression in normal human development. J Am Soc Nephrol. 2001;12:2175-2180. [PubMed] [Cited in This Article: ] |
| 11. | Montoli A, Colussi G, Massa O, Caccia R, Rizzoni G, Civati G, Barbetti F. Renal cysts and diabetes syndrome linked to mutations of the hepatocyte nuclear factor-1 beta gene: description of a new family with associated liver involvement. Am J Kidney Dis. 2002;40:397-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Bingham C, Hattersley AT. Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1beta. Nephrol Dial Transplant. 2004;19:2703-2708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Coffinier C, Barra J, Babinet C, Yaniv M. Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev. 1999;89:211-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829-1838. [PubMed] [Cited in This Article: ] |
| 15. | Shih DQ, Bussen M, Sehayek E, Ananthanarayanan M, Shneider BL, Suchy FJ, Shefer S, Bollileni JS, Gonzalez FJ, Breslow JL. Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet. 2001;27:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 327] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Kitanaka S, Miki Y, Hayashi Y, Igarashi T. Promoter-specific repression of hepatocyte nuclear factor (HNF)-1 beta and HNF-1 alpha transcriptional activity by an HNF-1 beta missense mutant associated with Type 5 maturity-onset diabetes of the young with hepatic and biliary manifestations. J Clin Endocrinol Metab. 2004;89:1369-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Beckers D, Bellanné-Chantelot C, Maes M. Neonatal cholestatic jaundice as the first symptom of a mutation in the hepatocyte nuclear factor-1beta gene (HNF-1beta). J Pediatr. 2007;150:313-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Raile K, Klopocki E, Holder M, Wessel T, Galler A, Deiss D, Müller D, Riebel T, Horn D, Maringa M. Expanded clinical spectrum in hepatocyte nuclear factor 1b-maturity-onset diabetes of the young. J Clin Endocrinol Metab. 2009;94:2658-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Gonc EN, Ozturk BB, Haldorsen IS, Molnes J, Immervoll H, Raeder H, Molven A, Søvik O, Njølstad PR. HNF1B mutation in a Turkish child with renal and exocrine pancreas insufficiency, diabetes and liver disease. Pediatr Diabetes. 2012;13:e1-e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Roelandt P, Antoniou A, Libbrecht L, Van Steenbergen W, Laleman W, Verslype C, Van der Merwe S, Nevens F, De Vos R, Fischer E. HNF1B deficiency causes ciliary defects in human cholangiocytes. Hepatology. 2012;56:1178-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Lawrence C, Fryer JG, Karlberg P, Niklasson A, Ericson A. Modelling of reference values for size at birth. Acta Paediatr Scand Suppl. 1989;350:55-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Kohsaka T, Yuan ZR, Guo SX, Tagawa M, Nakamura A, Nakano M, Kawasasaki H, Inomata Y, Tanaka K, Miyauchi J. The significance of human jagged 1 mutations detected in severe cases of extrahepatic biliary atresia. Hepatology. 2002;36:904-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Bellanné-Chantelot C, Clauin S, Chauveau D, Collin P, Daumont M, Douillard C, Dubois-Laforgue D, Dusselier L, Gautier JF, Jadoul M. Large genomic rearrangements in the hepatocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes. 2005;54:3126-3132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 24. | Chen YZ, Gao Q, Zhao XZ, Chen YZ, Bennett CL, Xiong XS, Mei CL, Shi YQ, Chen XM. Systematic review of TCF2 anomalies in renal cysts and diabetes syndrome/maturity onset diabetes of the young type 5. Chin Med J (Engl). 2010;123:3326-3333. [PubMed] [Cited in This Article: ] |
| 25. | Faguer S, Decramer S, Chassaing N, Bellanné-Chantelot C, Calvas P, Beaufils S, Bessenay L, Lengelé JP, Dahan K, Ronco P. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 2011;80:768-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, van’t Hoff W. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2009;20:1123-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, Huart A, Schanstra JP, Calvas P, Decramer S. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Müller D, Klopocki E, Neumann LM, Mundlos S, Taupitz M, Schulze I, Ropers HH, Querfeld U, Ullmann R. A complex phenotype with cystic renal disease. Kidney Int. 2006;70:1656-1660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |