Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2323
Peer-review started: April 27, 2014
First decision: May 27, 2014
Revised: July 22, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: February 28, 2015
AIM: To investigate the role of profilin-1 (PFN1) in gastric cancer and the underlying mechanisms.
METHODS: Immunohistochemical analysis, quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot were performed to detect PFN1 expression in clinical gastric carcinoma and adjacent tissues, and the association of PFN1 expression with patient clinicopathological characteristics was analyzed. PFN1 was knocked down to investigate the role of this protein in cell proliferation and metastasis in the SGC-7901 cell line. To explore the underlying mechanisms, the expression of integrin β1 and the activity of focal adhesion kinase (FAK) and the downstream proteins extracellular-regulated kinase (ERK)1/2, P38 mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), AKT and mammalian target of rapamycin (mTOR) were measured through Western blot or qRT-PCR analysis. Fibronectin (FN), a ligand of integrin β1, was used to verify the correlation between alterations in the integrin β1/FAK pathway and changes in tumor cell aggressiveness upon PFN1 perturbation.
RESULTS: Immunohistochemical, Western blot and qRT-PCR analyses revealed that PFN1 expression was higher at both the protein and mRNA levels in gastric carcinoma tissues compared with the adjacent tissues. In addition, high PFN1 expression (53/75, 70.4%) was correlated with tumor infiltration, lymph node metastasis and TNM stage in gastric cancer, but not with gender, age, location, tumor size, or histological differentiation. In vitro experiments showed that PFN1 knockdown inhibited the proliferation of SGC-7901 cells through the induction G0/G1 arrest. Silencing PFN1 inhibited cell migration and invasion and down-regulated the expression of matrix metalloproteinase (MMP)-2 and MMP9. Moreover, silencing PFN1 reduced the expression of integrin β1 at the protein level and inhibited the activity of FAK, and the downstream effectors ERK1/2, P38MAPK, PI3K, AKT and mTOR. FN-promoted cell proliferation and metastasis via the integrin β1/FAK pathway was ameliorated by PFN1 silencing.
CONCLUSION: These findings suggest that PFN1 plays a critical role in gastric carcinoma progression, and these effects are likely mediated through the integrin β1/FAK pathway.
Core tip: The expression of profilin-1 (PFN1) has been detected in many types of human cancers and has also been associated with tumor malignancy. However, the role of PFN1 in gastric carcinoma is unclear. The results of the present study suggest an important role for PFN1 in gastric cancer. PFN1 is overexpressed in gastric cancer, associated with tumor infiltration, lymph node metastasis and TNM stage. Furthermore, we demonstrated that PFN1 silencing inhibits gastric cancer cell proliferation, migration and invasion through the integrin β1/focal adhesion kinase pathway.
-
Citation: Cheng YJ, Zhu ZX, Zhou JS, Hu ZQ, Zhang JP, Cai QP, Wang LH. Silencing profilin-1 inhibits gastric cancer progression
via integrin β1/focal adhesion kinase pathway modulation. World J Gastroenterol 2015; 21(8): 2323-2335 - URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2323.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2323
Gastric cancer is the second most common cause of cancer deaths worldwide[1]. Traditional therapies for gastric cancer include surgical resection and chemotherapy, but these therapies are non-curative for patients diagnosed with advanced gastric cancer. Therefore, more effective treatments are urgently needed for this aggressive malignancy. The molecular mechanisms of gastric cancer progression should be clarified to identify potential therapeutic markers. The dysregulation of the actin cytoskeleton is a hallmark of tumor transformation, which is controlled through actin-binding proteins that regulate the nucleation, branching, elongation, bundling, severing, and capping of the actin filament[2,3]. Thus, identifying actin-binding proteins might facilitate the discovery of novel targets for gastric cancer therapies.
Profilin-1 (PFN1), an important actin-binding protein, was first identified more than 30 years ago in the calf thymus[4]. A biological role for PFN1 in cancer has recently emerged, and this protein has been implicated in almost every cellular function, including proliferation, survival, motility, endocytosis and membrane trafficking, mRNA splicing and gene transcription[5]. It has been recently shown that PFN1 is underexpressed in some human solid cancers, such as breast, pancreas, and liver carcinomas, while the overexpression of this protein can inhibit proliferation and migration of these cancer cells, suggesting that PFN1 might be a tumor suppressor protein[6-8]. In contrast, some reports have shown PFN1 overexpression in other cancers, such as renal cell carcinoma and laryngeal carcinoma[9-16]. These differences suggest that PFN1 might be involved in different tumorigenic mechanisms in different tissue types. However, few studies have demonstrated a role for PFN1 in gastric cancer.
Integrins are heterodimeric cell-surface molecules that associate the actin cytoskeleton to the cell membrane on one side and mediate cell-matrix interactions on the other side[11]. Actin-binding proteins mediate the adhesion of cells to extracellular matrices and cell survival through the association of integrins with the cortical actin cytoskeleton[12]. Recently, studies have reported that PFN1 facilitates staurosporine-triggered apoptosis through the regulation of the concentration of integrin β1 associated with the cytoskeleton on the cell surface[13]. However, the contribution of the integrin β1 downstream signaling pathway to the biological role of PFN1 has not been clarified.
In the present study, we examined PFN1 expression in gastric cancer tissues and gastric cancer cell lines and analyzed the influence of PFN1 expression on patient clinicopathological characteristics. Furthermore, PFN1 was knocked down to determine the role of this protein in the proliferation and metastasis of SGC-7901 cells. To explore the underlying mechanisms, the expression of integrin β1 and the activity of focal adhesion kinase (FAK) and downstream proteins extracellular-regulated kinase (ERK)1/2, P38 mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), AKT and mammalian target of rapamycin (mTOR) were measured through Western blot or quantitative real-time polymerase chain reaction (qRT-PCR). Moreover, fibronectin (FN), a ligand of integrin β1, was used to verify the association between alterations in the integrin β1/FAK pathway and changes in tumor cell aggressiveness upon PFN1 perturbation.
This study was approved by the Ethics Committee of the Scientific and Ethical Committee of Second Military Medical University, and informed consent form was obtained from all participants.
All tissue specimens, including 75 gastric cancer tissues and the corresponding non-plastic gastric mucosal tissues for immunohistochemistry and another 30 frozen gastric cancer and paired adjacent normal tissues (not included in immunohistochemistry tissues) for qRT-PCR and Western blot, were obtained from patients during surgery at Shanghai Changzheng Hospital. All noncancerous human gastric tissue samples were obtained from gastrectomies of adjacent gastric cancer margins greater than 5 cm. All patients underwent surgical resection and were not treated with neoadjuvant chemotherapy or radiotherapy.
Immunohistochemical staining was performed using the EnVision system (Dako Carpinteria, CA, United States). TMA sections were submerged in citrate buffer (pH 6.0) and microwaved at 99 °C for 10 min for antigenic retrieval. PFN1 expression was detected using a primary antibody against PFN1 (1:200, ab133529, Epitomics, United States), and the immunostaining was evaluated as previously described[14]. Two pathologists blinded to the findings of the other researcher independently analyzed the immunohistochemical staining. A semiquantitative estimation of the staining was conducted using a composite score obtained from the product of the staining intensity and relative abundance of positive cells. The intensity was graded as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). The abundance of positive cells was graded from 0 to 4 (0, < 5% positive cells; 1, 5%-25%; 2, 26%-50%; 3, 51%-75%; and 4, > 75%). A composite score in cancer tissues greater than that in normal tissues was considered high expression, and a composite score in cancer tissues less than or equal to that in normal tissues was considered low expression.
The following human gastric carcinoma cell lines were used: AGS purchased from the American Type Culture Collection; SH-10-TC obtained from the Riken Bio Resource Center; MKN28, SGC-7901, BGC-803, BGC-823, N87 and gastric epithelial cells (GES) provided by the Institute of Biochemistry and Cell Biology of the Chinese Academy of Science. All cells were maintained in RPMI 1640 (Biowest) medium supplemented with 10% fetal bovine serum (FBS; Biowest), 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, 1 mmol/L sodium pyruvate, with or without 10 μg/mL FN (Sigma) at 37 °C in 5% CO2. The cells were passaged using trypsin-EDTA (0.05% trypsin and 0.53 mmol/L tetrasodium EDTA).
The siRNAs against human PFN1 were chemically synthesized at Shanghai GenePharma Co., Ltd. The siRNA sequences for the indicated genes were designed as previously described[16]. According to the manufacturer’s specifications, the cells were transfected with siRNA-PFN1 in 6-well plates using Lipofectamine 2000 (Invitrogen, Gaithersburg, MD). Briefly, before transfection, 2.0 × 105 cells were grown to 30%-50% confluence in 2 mL of growth medium without antibiotics. The Lipofectamine 2000, diluted in Opti-MEM I Reduced Serum medium (Gibco), was used to supplement the dsRNA mixture, and the mixture was incubated for 25 min. Subsequently, 6 μL of 20 μmol/L dsRNA formulated with 8 μL of Lipofectamine 2000 was added to a final volume of 2 mL Opti-MEM I Reduced Serum medium. The cells were incubated at 37 °C in a CO2 incubator for 24-48 h. The medium was changed after 6 h.
The harvested cells and human tissue specimens were lysed in a buffer containing 50 mmol/L Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, phosphatase inhibitors (100 mmol/L Na3VO4, 10 mmol/L NaF) and protease inhibitor (1 mmol/L phenyl methylsulphonyl fluoride) to obtain whole cell lysates. The insoluble tissue debris was removed through centrifugation at 13000 ×g at 4 °C for 15 min. The supernatant was collected, and the protein concentration was quantified using a protein assay reagent (BCA, Beyotime, Shanghai). The proteins were separated through SDS-PAGE, transferred to a nitrocellulose membrane and incubated with antibodies against total and phosphorylated FAK (pY397; Cell Signaling Technology, United States), total and phosphorylated AKT (Ser473; Cell Signaling Technology), total and phosphorylated mTOR (Ser2448; Cell Signaling Technology), total and phosphorylated PI3K (Tyr485 and Tyr199; Cell Signaling Technology), total and phosphorylated ERK1/2 (Thr202,Thr204; Cell Signaling Technology), PFN1 (Abcam, Cambridge, United Kingdom), matrix metalloproteinase (MMP)-2 (Abcam), MMP9 (Abcam), and integrin β1 (Abcam) at 4 °C overnight. The immunocomplexes were visualized using a horseradish peroxidase conjugated antibody followed by incubation with a chemiluminescence reagent (Millipore, Billerica, MA, United States) and exposure to a photographic film. The Western blot was quantified and analyzed using Quantity One software.
Total RNA was isolated from frozen tissues, and the cells were treated with TRIzol (Takara, Dalian, China). The RNA quality (A260/A280 ratio) and quantity were determined using a standard spectrophotometer. One microgram of total RNA was used for cDNA synthesis using the RevertAidTM First Strand cDNA Synthesis Kit 1622 (Fermentas, Vilnius, Lithuania) according to the manufacturer’s instructions. Appropriate forward and reverse primers were used in the reverse transcription-polymerase chain reactions (RT-PCRs) for cDNA amplification to detect the transcripts of interest. The primer sequences for GAPDH, PFN1 and integrin β1 have been previously described[13,16]. The following PCR conditions were used for the amplification: 94 °C for 10 min, followed by 40 cycles of 94 °C for 30 s, 55-58 °C for 30 s, and 72 °C for 45 s, and a final cycle at 72 °C for 10 min. qRT-PCR was conducted using a 7300 Real-time PCR System (Applied Biosystems). Standard curves were plotted for each optimized assay, to generate a linear plot of the threshold cycle (Ct) against log (dilution). The concentration of each target was quantified based on the concentration obtained from the standard curve and presented in arbitrary units. The quantity of each target was normalized against the quantity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
The cells (1 × 104/mL) were plated onto 96-well plates. At 24, 48 and 72 h post-transfection with PFN1 siRNA, cell viability was determined using the cell counting kit-8 (CCK-8) assay (RS Biotechnology, Shanghai) according to the manufacturer’s protocol. The experiments were performed in triplicate.
Colony formation assays were performed as previously described[15]. Briefly, the cells (500 per well) were cultured in 6 well plates for 2 wk. The colonies were counted and photographed using Quantity One software. All experiments were performed in triplicate.
SGC-7901 cells were transfected with PFN1 siRNA, NC and controls and harvested at 48 h post-transfection. The collected cells were fixed in 70% ethanol at 4 °C overnight. The cells were subsequently labeled with propidium iodide (PI) in the presence of RNase A. The fractions of cells in the G0/G1, S and G2/M phases were analyzed using flow cytometry. The experiments were performed in triplicate.
Transwell assays were performed using polycarbonate transwell filters (Corning, 8 μm) placed over the bottom chambers, which were filled with culture medium containing 20% FBS. Briefly, 1 × 105 cells in 0.5 mL of serum-free RPMI 1640 medium were placed in the upper chamber, and the lower chamber was loaded with 0.8 mL of medium containing 20% FBS. After 24 h, the cells on the upper surface of the well were removed, and the cells on the lower surface were fixed in cold methanol and stained with 0.4% crystal violet. For each experiment, the number of transmigrated cells in five random fields on the underside of the filter were counted and photographed, and three filters were independently analyzed.
The invasion assays were performed using BD Matrigel invasion chambers (BD, 8 μm). Briefly, 1 × 105 cells in 0.5 mL of serum-free RPMI 1640 medium were placed in the upper chamber, and the lower chamber was loaded with 0.8 mL of medium containing 20% FBS. After 24 h, the cells on the upper surface of the well were removed, and the cells on the lower surface were fixed in cold methanol and stained with 0.4% crystal violet. For each experiment, the number of transmigrated cells in five random fields on the underside of the filter were counted and photographed, and three filters were independently analyzed.
Data are expressed as mean ± SD. Statistical analyses were performed using Student’s t-test and analysis of variance. Pearson’s χ2 test was applied to examine the relationships between different variables. A P-value less than 0.05 was considered statistically significant.
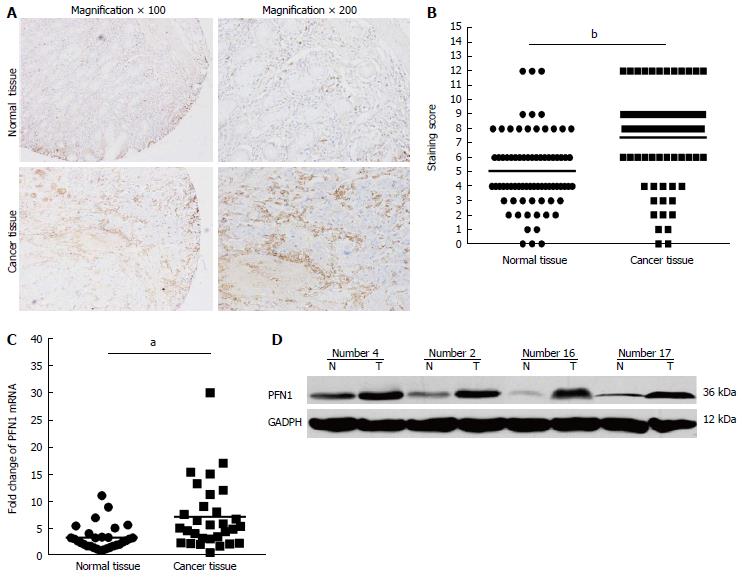
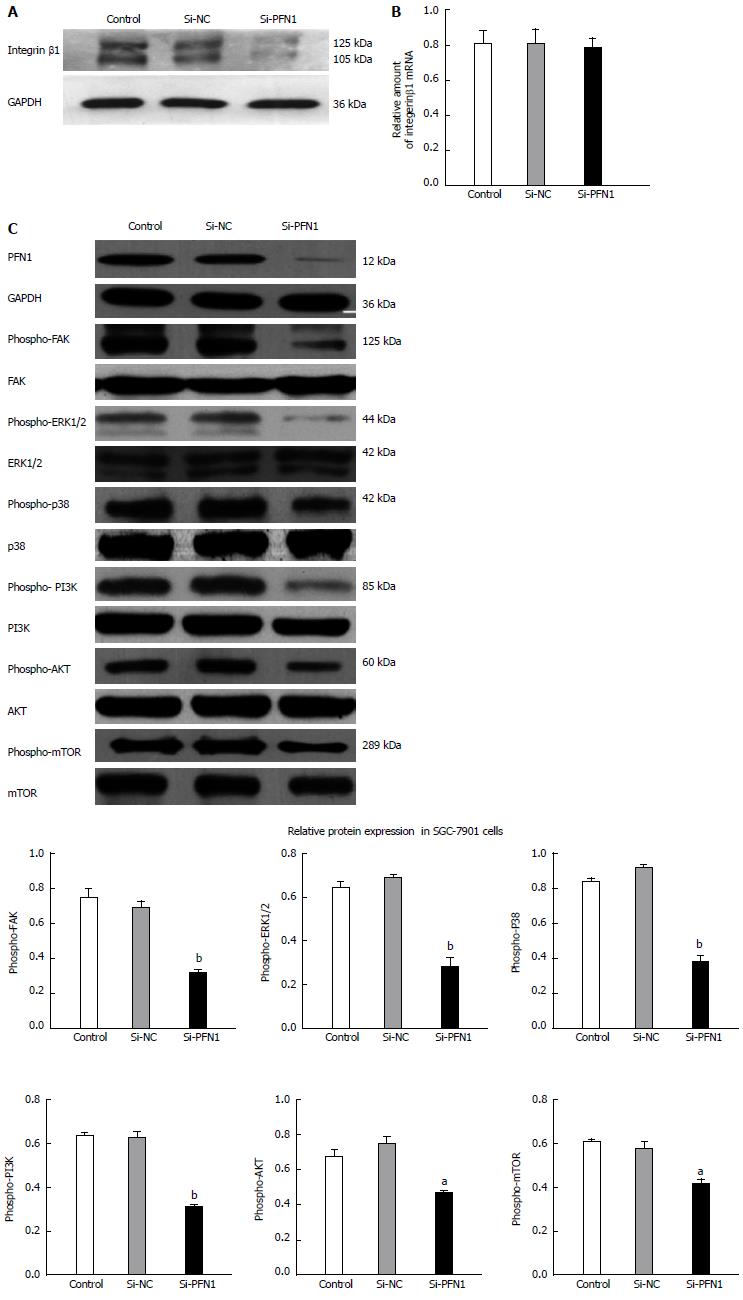
All tissue microarray block sections used in this study contained both normal and malignant epithelium. Figure 1A shows representative images of PFN1 immunostaining in gastric cancer tissue samples. A semiquantitative estimation of the staining was represented as a composite score obtained from the product of the staining intensity and relative abundance of positive cells. Among the 75 paired gastric cancer samples, 53 (70.4%) cancer tissue samples showed higher PFN1 expression than the matched adjacent normal tissues. Moreover, the staining score (P < 0.01) showed that PFN1 expression was higher in gastric cancer tissues than in the adjacent normal tissues (Figure 1B). These results were consistent with those obtained from the qRT-PCR and Western blot analyses using another 30 tissue sample pairs (Figure 1C, D). Furthermore, the associations between PFN1 expression and the clinicopathological factors of gastric cancer are shown in Table 1. High PFN1 expression was associated with tumor infiltration (P < 0.05), lymph node metastasis (P < 0.05) and TNM stage (P < 0.05), but not with gender, age, location, tumor size, or histological differentiation (P > 0.05).
| Parameter | Cases | PFN1 expression | P value | |
| High expression | Low expression | |||
| Gender | 0.073 | |||
| Male | 50 | 32 (60.4) | 18 (81.8) | |
| Female | 25 | 21 (39.6) | 4 (18.2) | |
| Age (yr) | 0.572 | |||
| > 60 | 51 | 35 (66) | 16 (72.7) | |
| ≤ 60 | 24 | 18 (34) | 6 (27.3) | |
| Location | 0.457 | |||
| Cardia | 15 | 9 (60) | 6 (40.0) | |
| Corpus | 26 | 18 (69.2) | 8 (30.8) | |
| Antrum | 34 | 26 (76.5) | 8 (23.5) | |
| Size (diameter) (cm) | 0.601 | |||
| < 6 | 41 | 30 (56.6) | 11 (50.0) | |
| ≥ 6 | 34 | 23 (43.4) | 11 (50.0) | |
| Differentiation | ||||
| I and II | 33 | 21 (39.6) | 12 (54.5) | 0.236 |
| III and IV | 42 | 32 (60.4) | 10 (45.5) | |
| Invasion depth | ||||
| T0-T2 | 19 | 10 (18.9) | 9 (41.0) | 0.046 |
| T3-T4 | 56 | 43 (81.1) | 13 (59.0) | |
| Nodal metastasis | ||||
| Negative | 27 | 15 (28.3) | 12 (54.5) | 0.038 |
| Positive | 48 | 38 (71.7) | 10 (45.5) | |
| TNM stage | ||||
| I and II | 29 | 16 (30.1) | 13 (59.0) | 0.036 |
| III and IV | 46 | 37 (69.9) | 9 (41.0) | |
| Total | 75 | 53 (70.4) | 22 (39.6) | |
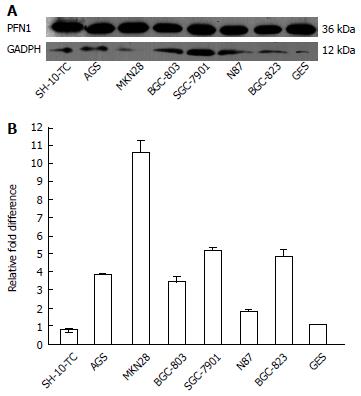
The expression of PFN1 in the gastric cancer epithelial cell lines AGS, MKN28, SGC-7901, BGC-823, N87, SH-10-TC, BGC-803 and GES was determined by Western blot and qRT-PCR. Among these cell lines, the gastric cancer epithelial cells exhibited relatively higher levels of PFN1 expression than the gastric epithelial cell lines (Figure 2). These results indicated that the expression of PFN1 in gastric cancer cells was relatively high, consistent with the levels detected in tissues.
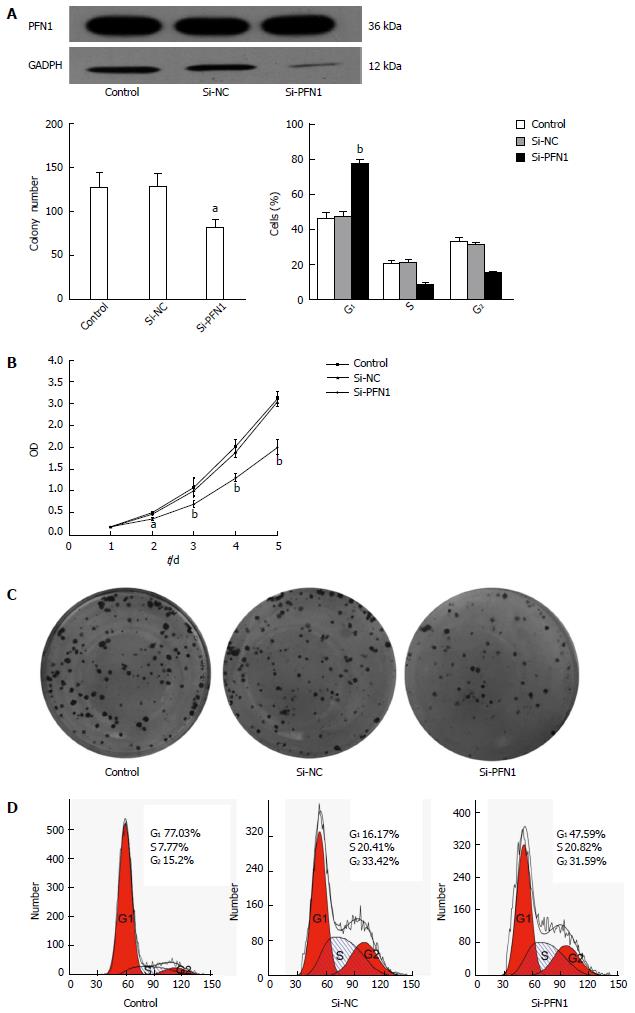
To address the potential role of PFN1 in the tumorigenesis of gastric cancer, SGC-7901 cells with relatively higher PFN1 expression were transfected with PFN1-siRNA. The down-regulated expression of PFN1 was evidenced through Western blot (Figure 3A). The effects of PFN1 knockdown on the proliferation of SGC-7901 cells were evaluated using CCK-8 kit. As shown in Figure 3B, silencing PFN1 significantly inhibited cell growth in PFN1-siRNA transfected cells relative to control and vector transfectants at 48 and 72 h (P < 0.05). However, significant differences were not observed at 24 h (P > 0.05). In addition, the colony formation assay revealed that the PFN1-siRNA transfected cells exhibited a significantly lower colony-forming efficiency (P < 0.05) (Figure 3C). Moreover, cell apoptosis and the cell cycle were assessed using flow cytometry to determine the mechanisms underlying the observed tumor suppression in response to PFN1 silencing. PFN1 silencing resulted in higher G0/G1 phase populations compared with the untreated control and NC-transfectants (Figure 3D), but no influence on cell apoptosis was observed (data not shown). Taken together, these results indicate that silencing PFN1 inhibits proliferation through the induction of G0/G1 arrest in SGC-7901 cells.
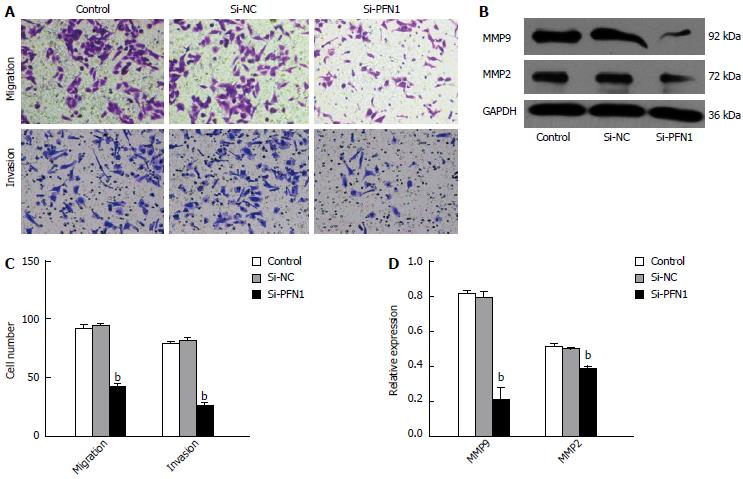
The migration of SGC-7901 cells was assessed using transwell migration assays. The micrographs obtained from a typical transwell experiment (Figure 4A) indicated that fewer cells transmigrated in the PFN1 siRNA-treated group compared with those in the untreated control and NC-transfected groups. Because MMPs are important mediators of tumor cell invasiveness and metastasis, we investigated the impact of gene silencing on MMP expression. The knockdown of PFN1 reduced MMP2 and MMP9 expression (Figure 4B). These results suggested that PFN1 meditates gastric cancer cell migration and invasion.
To elucidate the molecular mechanisms underlying tumor inhibition in response to PFN1 silencing, we assessed integrin β1 expression using Western blot and qRT-PCR. The results showed that PFN1 silencing did not affect the integrin β1 mRNA level (Figure 5B), but led to the down-regulation of integrin β1 protein expression (Figure 5A). The FAK signaling pathway plays an important role in the response to integrin-mediated cell proliferation, cell motility and migration. The expression of phospho-FAK and the downstream effectors phospho-ERK1/2, phospho-P38, phospho-PI3K and phospho-AKT were down-regulated after PFN1 silencing (Figure 5C). Because mTOR is a direct target of AKT, mTOR expression was also examined using Western blot, and the results showed that activities of these proteins were inhibited through PFN1 silencing (Figure 5C).
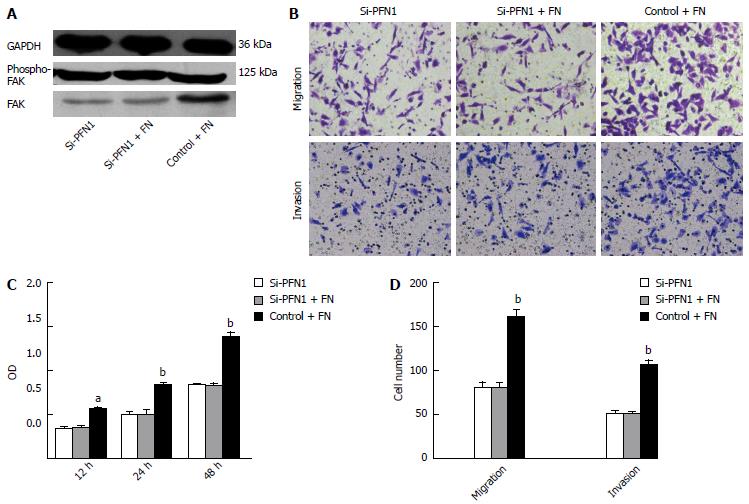
FN, an integrin β1 ligand, was used to verify the association between alterations in the integrin β1/FAK pathway and changes in tumor cell aggressiveness through PFN1 silencing. The activation of the FAK signaling pathway through FN via integrin β1 binding promoted cell proliferation, migration, and invasion in different cell types. The induction of FAK activity through FN was ameliorated after PFN1 silencing (Figure 6A). Moreover, the effect of FN on the cell proliferation, migration and invasion was ameliorated after PFN1 silencing (Figure 6B and C).
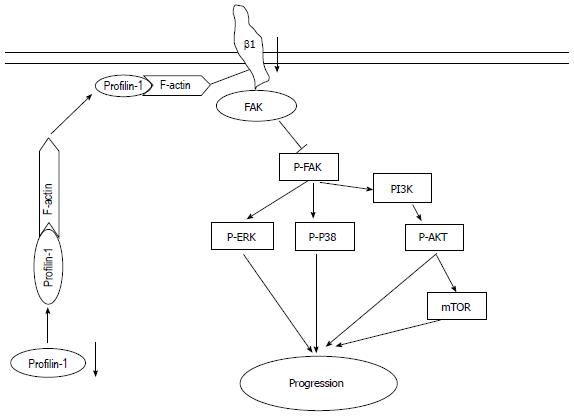
PFN1 is a 12-15 kDa protein that plays important roles in cellular functions. This protein regulates signaling-dependent actin polymerization via actin monomer binding at a ratio of 1:1 to form the profiling-actin complex, which participates in a variety of cellular functions, including growth and division, cell adhesion and motility, signal transduction and formation, and the maintenance of actin-binding protein-dependent cell morphology[17,18]. The results of the present study suggest that increased PFN1 expression promotes gastric cancer progression through stabilizing integrin β1 via changes in the FAK pathway.
The immunohistochemistry data revealed that PFN1 expression significantly increased from normal tissues to primary tumors. This result was also verified by qRT-PCR and Western blot in another set of paired gastric cancer tissues. We demonstrated that PFN1 is dysregulated in gastric cancer and gastric cancer cell lines, which is consistent with the findings of Tanaka et al[19]. In addition, the high expression of PFN1 was associated with tumor infiltration, lymph node metastasis and TNM stage. These results indicate that PFN1 plays an important role in gastric cancer progression.
To determine the biological function of this protein in gastric cancer, SGC-7901 cells with high PFN1 expression were subjected to PFN1 knockdown. The results of the cell proliferation and colony formation assays demonstrated that PFN1 silencing significantly inhibited cell growth. This result is consistent with association of PFN1 overexpression with TNM stage. In addition, PFN1 silencing also reduced cellular proliferation in other cancer cell lines, such as MCF7, HeLa, and SKOV3[20]. To further examine the function of this protein in gastric cancer, the effects of PFN1 on cell apoptosis and cell cycle distribution were analyzed using flow cytometry. The results showed that PFN1 silencing reduced the number of S- and G2-phase cells and increased the number of G0/G1-phase cells, indicating a G0/G1 arrest. However, cell apoptosis was not affected after PFN1 silencing (data not shown). These results suggest that PFN1 silencing inhibits the proliferation of gastric cancer cells through the induction of G0/G1 arrest.
In addition, PFN1 silencing significantly decreased gastric cancer cell migration and invasion. Consistently, blocking PFN1 decreased bladder cancer cell motility through reduced actin (F-actin) polymerization[21]. Furthermore, we observed that PFN1 silencing reduced the cellular expression of MMP2 and MMP9, two key enzymes involved in the degradation of the extracellular matrix (ECM). Because the ECM exerts biochemical and mechanical barriers to cell movement, the degradation of this scaffold is an important process in cancer cell metastasis[22]. The data presented here support these observations, suggesting that high PFN1 expression plays an essential role in the gastric cancer progression.
Indeed, the results of the present study showed that PFN1 silencing decreased integrin β1 at the protein level. A recent study suggested that PFN1 contributes to the quantity of integrin β1 linked to the cytoskeleton on the cell surface by promoting actin polymerization to increase the amount of F-actin[13]. Therefore, the alterations in integrin β1 expression at the protein level may reflect a decrease in the F-actin concentration after PFN1 silencing. Integrins including integrin β1 are non-kinase receptors, and their binding to the ECM and subsequent activation require kinases to initiate signal transduction. Integrin β1 signaling occurs primarily through the recruitment and activation of the tyrosine protein kinase FAK, and FAK promotes cancer cell proliferation and metastasis upon integrin β1 binding, which consequently results in the activation and auto-phosphorylation of FAK[23,24]. In the present study, we revealed that PFN1 silencing might inhibit the activation of FAK by down-regulating integrin β1 expression.
Activating FAK ensures successive signaling events, as this protein binds to the SH2 domain of PI3K, thereby transporting the catalytic subunit of PI3K to the membrane, where it catalyzes the phosphorylation of AKT[25]. mTOR is a direct target of AKT oncogenic signaling, involved in cell growth, tumorigenesis, and cell invasion in various types of cancers, including gastric cancer[26]. In addition, it can also activate the ERK1/2/P38MAPK signaling pathway by binding to ERK1/2 with a specific sequence[27]. Moreover, FAK inactivity leads to the inhibition of ERK1/2, P38MAPK, PI3K and AKT activity. Therefore, we examined changes in the expression of these molecules, and the results showed that the activity of the enzymes was inhibited after PFN1 silencing[28]. Previous studies have indicated that FAK/PI3K/AKT and FAK/MAPK are involved in the regulation of MMP2 and MMP9 activities in different cell types[29]. Consistently, MMP2 and MMP9 expression was down-regulated after PFN1 depletion. Taken together, these results suggest that PFN1 silencing might inhibit gastric cancer progression through the integrin β1/FAK signaling pathway.
Moreover, FN, an integrin β1 ligand, was used to verify the association between alterations in the integrin β1/FAK pathway and changes in tumor cell aggressiveness upon PFN1 perturbation. The integrin β1/FAK signaling pathway, activated through FN binding to integrin β1, promotes cell proliferation, migration, and invasion[30,31], and this effect can be ameliorated through the inhibition of the FAK pathway using an integrin β1 neutralizing antibody[31,32]. In the present study, this effect was also ameliorated through the down-regulation of integrin β1 protein expression via PFN1 silencing. Integrin β1 is a non-kinase receptor, and the subsequent activation of this receptor requires FAK to initiate signal transduction. In addition, the FN-mediated activation of FAK is ameliorated after PFN1 silencing through down-regulation of integrin β1 expression. Thus, the effect of FN on cell proliferation, migration and invasion could be ameliorated through PFN1 silencing via the integrin β1/FAK pathway. These results suggest that PFN1 silencing inhibits gastric cancer progression through the integrin β1/FAK pathway (Figure 7).
Traditionally, PFN1 has been considered indispensable for the pro-proliferation and pro-migration of cells through actin cytoskeleton remodeling via actin polymerization[32,33]. The data in present study are consistent with this role for PFN1; however, these results are different from those in other studies concerning cancers, such as breast[34], pancreas[35], and liver cancers[8]. These differences suggest that PFN1 might be involved in different tumorigenic mechanisms in different tissue types. In addition, the recent study reported that PFN1 had contrasting effects on early vs late steps of breast cancer metastasis, and the loss of PFN1 significantly inhibited the metastatic outgrowth of disseminated breast cancer cells, which had a completely different tumor microenvironment than the primary tumor[2]. These results suggested that this contradiction was critically influenced through signaling from the tumor microenvironment. Meditated through PFN1 and the ECM of tumor microenvironment, integrin β1 acts as a bridge connecting the actin cytoskeleton, and this interaction might play an important role in the tumor cell response to the loss of PFN1 expression. Consistently, blocking integrin signaling significantly inhibits metastatic outgrowth of breast cancer cells[2]. Therefore, profilin-1 silencing inhibits gastric cancer progression through integrin β1/FAK pathway modulation.
In conclusion, the present study underscores an important role for PFN1 in gastric cancer. PFN1 overexpression in gastric cancer is associated with tumor infiltration, lymph node metastasis and TNM stage. Furthermore, we demonstrated that PFN1 silencing inhibits gastric cancer progression through the integrin β1/FAK pathway.
The authors would like to thank Zheng-Wei Zhang for technical assistance.
Gastric cancer is the second most common cause of cancer deaths worldwide. Traditional therapies for gastric cancer include surgical resection and chemotherapy, but these therapies are non-curative for those diagnosed with advanced gastric cancer. Therefore, more effective treatments are urgently needed for this aggressive malignancy.
Recent studies have suggested that profilin-1 (PFN1) might be involved in different tumorigenic mechanisms in different tissue types. However, there are few studies concerning the role of PFN1 in gastric cancer.
This study underscores an important role for PFN1 in gastric cancer. PFN1 overexpression in gastric cancer is associated with tumor infiltration, lymph node metastasis and tumor-node-metastasis (TNM) stage. Furthermore, authors demonstrated that PFN1 silencing inhibits gastric cancer cell proliferation, migration and invasion through the integrin β1/focal adhesion kinase (FAK) pathway.
The findings in the present support the idea that PFN1 might be a novel target for gastric cancer therapy.
PFN1, an important actin-binding protein, was first identified more than 30 years ago in the calf thymus and this protein was considered to play an indispensable role in cell pro-proliferation and pro-migration through actin cytoskeleton remodeling via actin polymerization.
In this study, the authors demonstrated that profilin-1 plays important roles in gastric cancer, associated with tumor infiltration, lymph node metastasis and TNM stage. The authors concluded that PFN1 promotes the progression of gastric cancer via modulation of the integrin β1/FAK pathway. This article is concise and well organized.
P- Reviewer: Kim H, Liu YB, Pang XH, Wang ZW, Xu JJ S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 2. | Ding Z, Joy M, Bhargava R, Gunsaulus M, Lakshman N, Miron-Mendoza M, Petroll M, Condeelis J, Wells A, Roy P. Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis. Oncogene. 2014;33:2065-2074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Mouneimne G, Hansen SD, Selfors LM, Petrak L, Hickey MM, Gallegos LL, Simpson KJ, Lim J, Gertler FB, Hartwig JH. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell. 2012;22:615-630. [PubMed] [Cited in This Article: ] |
| 4. | Carlsson L, Nyström LE, Sundkvist I, Markey F, Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977;115:465-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 503] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Ding Z, Bae YH, Roy P. Molecular insights on context-specific role of profilin-1 in cell migration. Cell Adh Migr. 2012;6:442-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Janke J, Schlüter K, Jandrig B, Theile M, Kölble K, Arnold W, Grinstein E, Schwartz A, Estevéz-Schwarz L, Schlag PM. Suppression of tumorigenicity in breast cancer cells by the microfilament protein profilin 1. J Exp Med. 2000;191:1675-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Wittenmayer N, Jandrig B, Rothkegel M, Schlüter K, Arnold W, Haensch W, Scherneck S, Jockusch BM. Tumor suppressor activity of profilin requires a functional actin binding site. Mol Biol Cell. 2004;15:1600-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Wu N, Zhang W, Yang Y, Liang YL, Wang LY, Jin JW, Cai XM, Zha XL. Profilin 1 obtained by proteomic analysis in all-trans retinoic acid-treated hepatocarcinoma cell lines is involved in inhibition of cell proliferation and migration. Proteomics. 2006;6:6095-6106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Minamida S, Iwamura M, Kodera Y, Kawashima Y, Ikeda M, Okusa H, Fujita T, Maeda T, Baba S. Profilin 1 overexpression in renal cell carcinoma. Int J Urol. 2011;18:63-71. [PubMed] [Cited in This Article: ] |
| 10. | Masui O, White NM, DeSouza LV, Krakovska O, Matta A, Metias S, Khalil B, Romaschin AD, Honey RJ, Stewart R. Quantitative proteomic analysis in metastatic renal cell carcinoma reveals a unique set of proteins with potential prognostic significance. Mol Cell Proteomics. 2013;12:132-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163-180. [PubMed] [Cited in This Article: ] |
| 12. | Kim H, Sengupta A, Glogauer M, McCulloch CA. Filamin A regulates cell spreading and survival via beta1 integrins. Exp Cell Res. 2008;314:834-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Yao W, Yu X, Fang Z, Yin P, Zhao C, Li N, Wang L, Li Z, Zha X. Profilin1 facilitates staurosporine-triggered apoptosis by stabilizing the integrin β1-actin complex in breast cancer cells. J Cell Mol Med. 2012;16:824-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Liu K, Wang G, Ding H, Chen Y, Yu G, Wang J. Downregulation of metastasis suppressor 1(MTSS1) is associated with nodal metastasis and poor outcome in Chinese patients with gastric cancer. BMC Cancer. 2010;10:428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Sun X, Qiu JJ, Zhu S, Cao B, Sun L, Li S, Li P, Zhang S, Dong S. Oncogenic features of PHF8 histone demethylase in esophageal squamous cell carcinoma. PLoS One. 2013;8:e77353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Li L, Zhang Z, Wang C, Miao L, Zhang J, Wang J, Jiao B, Zhao S. Quantitative proteomics approach to screening of potential diagnostic and therapeutic targets for laryngeal carcinoma. PLoS One. 2014;9:e90181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 435] [Reference Citation Analysis (0)] |
| 17. | Rawe VY, Payne C, Schatten G. Profilin and actin-related proteins regulate microfilament dynamics during early mammalian embryogenesis. Hum Reprod. 2006;21:1143-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat Cell Biol. 2002;4:626-631. [PubMed] [Cited in This Article: ] |
| 19. | Tanaka M, Sasaki H, Kino I, Sugimura T, Terada M. Genes preferentially expressed in embryo stomach are predominantly expressed in gastric cancer. Cancer Res. 1992;52:3372-3377. [PubMed] [Cited in This Article: ] |
| 20. | Yap KL, Fraley SI, Thiaville MM, Jinawath N, Nakayama K, Wang J, Wang TL, Wirtz D, Shih IeM. NAC1 is an actin-binding protein that is essential for effective cytokinesis in cancer cells. Cancer Res. 2012;72:4085-4096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 655] [Reference Citation Analysis (0)] |
| 21. | Zoidakis J, Makridakis M, Zerefos PG, Bitsika V, Esteban S, Frantzi M, Stravodimos K, Anagnou NP, Roubelakis MG, Sanchez-Carbayo M. Profilin 1 is a potential biomarker for bladder cancer aggressiveness. Mol Cell Proteomics. 2012;11:M111.009449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Sampieri CL, de la Peña S, Ochoa-Lara M, Zenteno-Cuevas R, León-Córdoba K. Expression of matrix metalloproteinases 2 and 9 in human gastric cancer and superficial gastritis. World J Gastroenterol. 2010;16:1500-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | de Paula CA, Coulson-Thomas VJ, Ferreira JG, Maza PK, Suzuki E, Nakahata AM, Nader HB, Sampaio MU, Oliva ML. Enterolobium contortisiliquum trypsin inhibitor (EcTI), a plant proteinase inhibitor, decreases in vitro cell adhesion and invasion by inhibition of Src protein-focal adhesion kinase (FAK) signaling pathways. J Biol Chem. 2012;287:170-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kornberg LJ. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck. 1998;20:745-752. [PubMed] [Cited in This Article: ] |
| 25. | Hwangbo C, Kim J, Lee JJ, Lee JH. Activation of the integrin effector kinase focal adhesion kinase in cancer cells is regulated by crosstalk between protein kinase Calpha and the PDZ adapter protein mda-9/Syntenin. Cancer Res. 2010;70:1645-1655. [PubMed] [Cited in This Article: ] |
| 26. | Li M, Sun H, Song L, Gao X, Chang W, Qin X. Immunohistochemical expression of mTOR negatively correlates with PTEN expression in gastric carcinoma. Oncol Lett. 2012;4:1213-1218. [PubMed] [Cited in This Article: ] |
| 27. | Goel PN, Gude RP. Curbing the focal adhesion kinase and its associated signaling events by pentoxifylline in MDA-MB-231 human breast cancer cells. Eur J Pharmacol. 2013;714:432-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Chen YY, Liu FC, Chou PY, Chien YC, Chang WS, Huang GJ, Wu CH, Sheu MJ. Ethanol extracts of fruiting bodies of Antrodia cinnamomea suppress CL1-5 human lung adenocarcinoma cells migration by inhibiting matrix metalloproteinase-2/9 through ERK, JNK, p38, and PI3K/Akt signaling pathways. Evid Based Complement Alternat Med. 2012;2012:378415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Chan KC, Ho HH, Huang CN, Lin MC, Chen HM, Wang CJ. Mulberry leaf extract inhibits vascular smooth muscle cell migration involving a block of small GTPase and Akt/NF-kappaB signals. J Agric Food Chem. 2009;57:9147-9153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Meng XN, Jin Y, Yu Y, Bai J, Liu GY, Zhu J, Zhao YZ, Wang Z, Chen F, Lee KY. Characterisation of fibronectin-mediated FAK signalling pathways in lung cancer cell migration and invasion. Br J Cancer. 2009;101:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Park JH, Ryu JM, Han HJ. Involvement of caveolin-1 in fibronectin-induced mouse embryonic stem cell proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. J Cell Physiol. 2011;226:267-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14:461-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 33. | Yousif NG. Fibronectin promotes migration and invasion of ovarian cancer cells through up-regulation of FAK-PI3K/Akt pathway. Cell Biol Int. 2014;38:85-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Zou L, Ding Z, Roy P. Profilin-1 overexpression inhibits proliferation of MDA-MB-231 breast cancer cells partly through p27kip1 upregulation. J Cell Physiol. 2010;223:623-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Cheng H, Li J, Liu C, Yao W, Xu Y, Frank TS, Cai X, Shi S, Lu Y, Qin Y. Profilin1 sensitizes pancreatic cancer cells to irradiation by inducing apoptosis and reducing autophagy. Curr Mol Med. 2013;13:1368-1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |